DOI: https://doi.org/10.56669/MMRA3791
ABSTRACT
Trichoderma is widely used in agriculture due to its known biocontrol mechanisms. By colonizing plants, either as an endophyte or on their roots, Trichoderma has developed the ability to interact with the plants and provide a variety of benefits to its hosts. The complexity of this plant-microbe association has led to a great deal of interest in the study of Trichoderma, ranging from its ability to act as a plant growth promoter to its ability to activate plant defense mechanisms against biotic and abiotic stresses. This review discusses the ideal properties of Trichoderma. It highlights its potential in biocontrol of plant diseases and pests, enhancement of nutrient use efficiency and promotion of plant growth, stress tolerance and bioremediation of organic pollutants, pesticides and heavy metals in arable soils, which are beneficial multipurpose properties for sustainable agriculture. The effectiveness of different agricultural wastes in the production of Trichoderma biofertilizers and the industrial production procedures of Trichoderma biomass are discussed.
Keywords: Biocontrol agent; plant growth promotion; stress tolerance; bioremediation
INTRODUCTION
Intensive agricultural practices and climate change have resulted in a loss of biodiversity, a change in pest and pathogen distributions, and chemical contamination of soil, air, and water resources that negatively impact the agroecosystem as well as human health. As agricultural policies are being modified to reduce the use of synthetic chemicals, there is an increasing importance of plant-beneficial fungus Trichoderma in farm practices due to its increased use as a biological alternative to agrochemicals and intensified research linking it to sustainable agriculture (Woo et al., 2023).
Trichoderma is a genus of filamentous fungi that can feed on other fungi (mycotrophism). It is found in virtually all environments, including agricultural land, forest, mountain, grassland, desert, and fresh and marine waters (Harman et al., 2004). Moreover, they are saprophytes, capable of colonizing and degrading dead organic matter. Trichoderma species thrive in all biotopes and have a broad geographic distribution worldwide. Currently, more than 375 species of Trichoderma have been identified (Kubiak et al., 2023). Trichoderma species can reproduce asexually by producing conidia (anamorph form) or sexually by producing ascospores that develop into fruiting bodies (teleomorph form). Additionally, they produce thick-walled spores, called chlamydospores, which help them survive in harsh environmental conditions (Harman et al., 2004). Trichoderma possesses strong cellulolytic and hemicellulolytic properties that allow it to accelerate the hydrolysis of polysaccharides in the cell wall of plant debris (Kumar et al., 2023). This is one of the main characteristics that favor their key application in mass production or industrial production (Kumar et al., 2023). In addition, Trichoderma species produce lignin-modifying enzymes that partially degrade this compound, which reduces its inhibitory effect on microorganisms (Kubiak et al., 2023).
As the challenges in overcoming problems in the agricultural sector have increased significantly in recent years, sustainable strategies with biological control methods are necessary. The versatile role of Trichoderma in sustainable agriculture makes it an excellent choice (Woo et al., 2023). Research interest has therefore expanded to a comprehensive analysis of the versatile properties of Trichoderma as plant-beneficial fungi for applications and improvements in agricultural production. This research interest should be aligned with the ongoing changes in agricultural policy and management and the growing concern for future sustainability.
The most important prerequisite for the commercialization of Trichoderma as a biocontrol or biofertilizer product is its ability for mass production through the economic output of the most significant number of efficient propagules (microsclerotia, conidia, and chlamydospores) in the shortest time (Fravel, 2005; Das and Kim, 2024). The main limitation for commercialization of the end product is the increased production cost due to expensive substrate or carrier materials (Fravel, 2005). However, there has been a growing interest in utilizing agricultural waste to produce biofertilizers or biocontrol products, which can not only promote sustainable agriculture and bring economic benefits to farmers but also reduce the environmental impacts associated with waste disposal. This review highlights the diverse role of Trichoderma in environmentally sustainable agriculture as well as the effectiveness of various agro-wastes in producing Trichoderma biomass.
Multifaceted role of Trichoderma in eco-sustainable agriculture
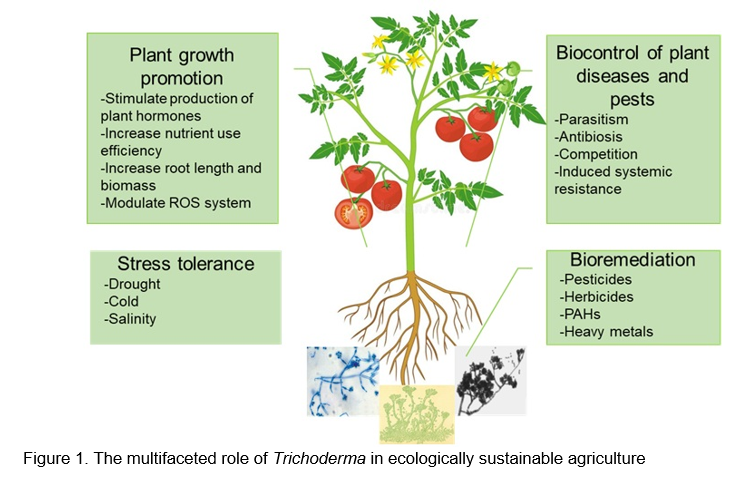
Trichoderma species are widely used as a key component in soil amendments, soil integrators, biostimulants, bioprotectants, biofertilizers, biodegraders, and bioremediators (Woo et al., 2023). They are particularly of interest to agriculture as biocontrol agents of phytopathogens, even though their many benefits have received considerable attention (Guzman-Guzman et al., 2023). Trichoderma uses direct competition and antagonism, especially in the rhizosphere, where it alters the composition of other microbes and their interactions with them. It has developed the ability to interact with plants through colonization, either on the roots or as an endophyte, and to provide a variety of diverse benefits to its host (Woo et al., 2023). Trichoderma has attracted a lot of interest in research because of its intricate relationship with plants and microorganisms, including its potential to stimulate plant growth, prime local and systemic defenses against biotic and abiotic stresses, and trigger transcriptional memory that affects future plant responses (Woo et al., 2023). Below we have discussed the diverse role of Trichoderma in biocontrol of plant diseases and pests, enhancement of nutrient use efficiency and plant growth promotion, stress tolerance, bioremediation, and above all its ideal characteristics in eco-sustainable agriculture. Figure 1 depicts the multifaceted role of Trichoderma in ecologically sustainable agriculture.
Biocontrol of plant diseases and pests
Since the 1920s, the widespread soil-dwelling fungi Trichoderma has been known to produce antibiotics and parasitize other fungi, which allows them to function as biocontrol agents against a variety of phytopathogens (Harman et al., 2004). It was later discovered that the induction of disease resistance was their primary method of protecting plants (Harman et al., 2004; Abdullah et al., 2021). Trichoderma uses both indirect and direct methods to control plant pathogens. Direct mechanisms involve mycoparasitism and coiling, while indirect mechanisms involve challenges for nutrients and space, acquired resistance, and antibiosis (Woo et al., 2023). The type of Trichoderma strains, the pathogen that is being repelled, including its host, and the ecological context all affect how effective these mechanisms are in the biocontrol strategy (Woo et al., 2023). A variety of pathogenic microorganisms that affect plants have been documented to be controlled by Trichoderma, including bacteria (Pseudomonas, Xanthomonas and Clavibacter), fungi (Fusarium, Botrytis, Colletotrichum, Erysiphe, Magnaporthe, Sclerotinia, Verticillium, Curvularia, Colletotrichum, Alternaria, Rhizoctonia, Athelia, Armillaria, Ustilago, Puccinia), oomycetes (Pythium and Phytophthora), and at least one virulent virus (green mottle mosaic virus) (Harman et al., 2004; Woo et al., 2023). The biocontrol of different Trichoderma species against plant pathogens is shown in Table 1.
Table 1. Biocontrol of different Trichoderma species against plant pathogens
|
Plant Disease
|
Crop
|
Causal agent
|
Trichoderma spp.
|
Reference
|
|
Bacterial wilt
|
Tomato (Solanum lycopersicum)
|
Ralstonia solanacearum
|
T. asperellum
|
Konappa et al., 2018
|
|
Fungal wilt
|
Tomato
|
Fusarium oxysporum
|
T. asperellum
|
El Komy et al., 2015
|
|
|
Melon (Cucumis melo)
|
F. oxysporum
|
T. harzianum
|
Bernal-Vicente et al., 2009
|
|
Leaf spot
|
Tomato
|
Xanthomonas euvesicatoria
|
T. harzianum
T. strigosum
|
Fontenelle et al., 2011
|
|
|
Cucumber (Cucumis sativus)
|
Pseudomonas syringae
|
T. harzianum
T. strigosum
|
Fontenelle et al., 2011
|
|
|
Sugar beet (Beta
vulgaris)
|
Cercospora
beticola
|
T. hermatum
|
Galletti et al., 2008
|
|
Damping off
|
Cucumber
|
Pythium sp.
|
T. harzianum
|
Paulitz et al., 1990
|
|
|
Sugar beet (Beta vulgaris)
|
Rhizoctonia solani
|
T. harzianum
|
Lewis and Papavizas, 1987
|
|
|
Cotton (Gossyphtm hirsutum)
|
Rhizoctonia solani
|
T. hamatum
|
Lewis and Papavizas, 1987
|
|
|
Cotton
|
Pythium aphanidermatum
|
T. virens
|
Howell, 2002
|
|
Root rot
|
Soybean (Glycine max)
|
Pythium arrhenomanes
|
T. viride
|
John et al., 2010
|
|
|
Corn (Zea mays)
|
Fusarium oxysporum
|
T. viride
|
John et al., 2010
|
|
|
Bean (Phaseolus
vulgaris)
|
Rhizoctonia
solani
|
T. asperellum
|
Asad et al., 2014
|
|
|
Pepper plants (Capsicum annuum)
|
Rhizoctonia solani
|
T. harzianum
|
Ahmed et al., 2003
|
|
|
Eggplant (Solanum melongena)
|
Macrophomina phaseolina
|
T. harzianum
|
Ramezani, 2008
|
|
Stalk rot
|
Maize (Zea
mays)
|
Fusarium
graminearum
|
T. asperellum
|
Li et al., 2016
|
|
Fruit rot
|
Chili (Capsicum annuum)
|
Alternaria tenuis
|
T. harzianum
|
Begum et al., 2010
|
|
|
Tomato
|
Rhizoctonia solani
|
T. viride, T. virens, T. harzianum
|
Amin and Razdan, 2010
|
|
Head blight
|
Wheat and other small grain cereals (Triticum
aestivum)
|
Fusarium graminearum,
F. culmorum
|
T. gamsii
|
Matarese et al., 2012
|
|
Sheath blight
|
Rice (Oryza sativa)
|
Rhizoctonia solani
|
T. harzianum
|
Naeimi et al., 2010
|
|
Blossom blight
|
Alfalfa (Medicago sativa)
|
Sclerotinia sclerotiorum
|
T. atroviride
|
Li et al., 2005
|
It is relatively complex to control plant diseases caused by bacteria. The use of biocontrol agents is effective at preventing bacterial pathogens and is safer for the environment than chemical bactericides. It was shown that Trichoderma inhibited the growth and survival of Ralstonia. This Gram-negative bacterium causes disease in tomato plants, due to the secretion of lysosime, viridiofungin, and trichokonin (Yan et al., 2021). Additionally, the application of Trichoderma asperellum suppressed bacterial wilt produced by the soilborne bacterium Ralstonia solanacearum, which in turn reduced the disease incidence while simultaneously improving plant growth and yield (Konappa et al., 2018). This was accomplished by increasing the total phenolic contents in plants and inducing the highest level of defense enzyme activities, including peroxidase (POX), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL), β-1,3-glucanase (Konappa et al., 2018). Biocontrol of bacterial phytopathogens is also illustrated by the induction of resistance by Trichoderma in tomato plants against Xanthomonas euvesicatoria (the causative agent of bacterial spot) and in cucumber plants against angular leaf spot caused by Pseudomonas syringae pv. Lachrymans (Fontenelle et al., 2011). Through a variety of mechanisms, such as lignification, the synthesis of phytoalexins, pathogenesis-related proteins, and secondary metabolites with antimicrobial properties, Trichoderma can protect plants from bacterial pathogens (Kumar et al., 2023).
In addition to plant diseases caused by bacteria, fungi are also often associated with plant diseases and crop damage, resulting in significant losses in agricultural production. Trichoderma has been found to have the ability to eliminate phytopathogenic fungi through various mechanisms, such as mycoparasitism, antibiosis, competition, production of antibiotics and other antifungal compounds, and induced systemic resistance (Kumar et al., 2023).
The process by which one fungus parasitizes another fungus (the host) is called mycoparasitism, and it is one of the key mechanisms of fungal antagonists (Harman et al., 2004). In addition to the pathogen cell wall penetration and host digestion, four sequential processes such as chemotaxis, identification, attachment, and wrapping have been identified as being involved in mycoparasitism (Kumar et al., 2023). Trichoderma koningii colonized injured or infected onion root tissues as a secondary colonizer, reducing Sclerotium cepivorum infection by eliminating the hyphae, rather than invading healthy tissues (Metcalf and Wilson, 2001). Conversely, Trichoderma virens reduced the inoculum potential of several pathogenic fungal species in soil by not only parasitizing their hyphae but also penetrating and destroying some of their resting structures (Howell, 2006). The enzymatic breakdown of cell walls caused by hydrolytic enzymes (such as chitinase, 1,3-glucanase, and cellulase) produced by Trichoderma leads to the degradation of host tissues containing pathogenic organisms (Kumar et al., 2023). It was shown that treating cotton seedlings with T. virens reduced the pre-emergence of damping-off disease caused by Rhizopus oryzae (Howell, 2002).
Antibiosis is the phenomenon whereby a microbe uses secondary metabolites and low molecular weight compounds and antibiotics to prevent or inhibit another organism. Trichoderma synthesizes secondary metabolites such as pyrone, heterocyclic compounds, terpenoids, polyketides, etc. and produces specific low molecular weight compounds and antibiotics to kill plant pathogens (Kumar et al., 2023). The different species of Trichoderma produce different antibiotics; for example, T. viride produces mucortoxins A and B, mucorin, trichophyton, and mucorin; T. mucorin produces mucorin A and B; T. harzianum produces tricholongins BI and BII; T. koningii produces longibrachins and trichokonins; T. atroviride produces atroviridines A-C and neoatroviridines A-D, while other antibiotics and fungicidal compounds have been isolated from T. harzianum, T. koningii, T. aureoviride, T. virens, T. hamatum, and T. lignorum. The koninginin D produced by Trichoderma inhibits the growth of soil pathogens such as Phytophthora solani, Phytophthora middletonii, Phytophthora cinnamomi, Bipolaris sorokiniana, and Fusarium oxysporum (Dunlop et al., 1989). The viridins obtained from Trichoderma species such as T. viride, T. koningii, and T. virens inhibit the germination of spores of Colletotrichum lini, Botrytis allii, Penicillium expansum, Fusarium caeruleum, Stachybotry satra, and Aspergillus niger (Singh et al., 2005). The harzianic acid produced by T. harzianum has antimicrobial activity against Sclerotinia sclerotiorum, Rhizoctonia solani, and Pythium irregular (Manganiello et al., 2018).
Fungal pathogens can be naturally controlled by competition for nutrients (Kumar et al., 2023). Certain characteristics make Trichoderma more competitive than other microorganisms, including a faster growth rate and the ability to mobilize and utilize nutrients from soil and substrate. The saprophytic ability and inoculum potential of Trichoderma are influenced by four primary characteristics: (i) fast germination of fungal propagules and rapid hyphal growth toward nutrients, (ii) production of enzymes that interact with the carbon constituents of the host plant, (iii) secretion of growth inhibitor compounds (fungistatic and bacteriostatic), and (iv) tolerance of competing microorganisms containing fungistatic compounds (Woo et al., 2023). An important factor in the interaction between Trichoderma and plant pathogens is the competition for macro and micronutrients (Harman et al., 2004). Trichoderma has been shown to compete with plant pathogens for nutrients, primarily iron, nitrogen, and carbon (Kumar et al., 2023). Trichoderma species operate as a competitor that aids in the control of plant diseases by producing iron chelating compounds and siderophores that prevent rhizospheric bacteria from obtaining iron, ultimately resulting in the extinction of the disease (Oszust et al., 2020). Studies have also found that Trichoderma can compete with plant pathogens, including Colletotrichum sp., Botrytis sp., and Phytophthora sp., for both complex and simple carbon substrates (Oszust et al., 2020).
Trichoderma confers local or systemic disease resistance by triggering a host plant's defensive mechanism while preventing pathogens from multiplying and growing (Woo et al., 2023). There are generally two methods to achieve Trichoderma-induced plant disease resistance: one is to control the inducers or stimulants that trigger plant disease resistance responses; the other is to use the cell wall-degrading enzymes produced by Trichoderma to release oligosaccharides to cause plant disease resistance (Kumar et al., 2023). It was found that Trichoderma coated corn seeds significantly increased peroxidase activity and phenylalanine ammonia lyase activity, and they proved to be resistant to Curvularia leaf spot (Saravanakumar et al., 2016). An isolate of T. harzianum was reported to induce resistance in tomato plants to bacterial spot (Xanthomonas campestris pv. vesicatoria), reducing disease incidence by 69.32% after 14 days of inoculation (Saksirirat et al., 2009).
Enhancement of nutrient use efficiency and plant growth promotion
Trichoderma can solubilize insoluble minerals via various mechanisms including redox activity and chelating metabolites and plays an important role in soil nutrient cycling (Kashyap et al., 2017). The role of Trichoderma in solubilizing tricalcium phosphate and other phosphorus has been well studied, with results indicating improved phosphorus availability to plants (Saravanakumar et al., 2013). Enhanced availability of P and Fe has been shown with significant increases in plant biomass after Trichoderma harzianum colonized cucumber roots (Yedidia et al., 2001). When T. harzianum was applied to sugarcane, the availability of N, P, and K increased by 27, 65, and 44%, respectively (Singh et al. 2010). The application of T. harzianum together with other bioagents increased the content of N, P, K, Fe, and Mg in chickpea leaves (Kashyap et al., 2017). In comparison to the recommended doses of NPK, Trichoderma biofertilizer increased tomato growth, leaf greenness, and mineral contents (P, K, Ca, Mg, Cu, Fe, Mn, and Zn) in tomato roots. It also produced a 12.9% higher yield (Khan et al., 2016). Trichoderma seed biopriming can cut the amount of nitrogen needed by 30 to 50% for a variety of crops (Harman 2011). These studies suggest that Trichoderma biofertilizers could reduce the need for chemical fertilizers, making them a recommended approach for sustainable agriculture.
Trichoderma are excellent plant growth-promoting fungi (PGPF) as they can produce plant growth-promoting substances such as indoleacetic acid (IAA), auxin and harzianic acid (Contreras-Cornejo et al., 2014). T. virens and T. atroviride were found to produce plant hormones such as indoleacetic acid (IAA) and auxin, and when Trichoderma spp. were inoculated into an Arabidopsis plant, the root tip grew (Contreras-Cornejo et al., 2014). Yedidia et al. (2001) found that a cucumber plant inoculated with T. harzianum significantly increased root biomass and increased the concentrations of Cu, P, Fe, Zn, Mn and Na in the root. Some of the Trichoderma spp. that play an important role as PGPF are listed in Table 2.
Table 2. Inoculation effects of different Trichoderma spp. on plant growth and development
|
Trichoderma spp.
|
Plant
|
Effects
|
References
|
T. virens
|
Arabidopsis
thaliana
|
Produce the auxin-related compounds indole-3-acetic acid, indole-3-acetaldehyde, and indole-3-ethanol and enhance plant biomass production and lateral root development
|
Contreras-Cornejo et al., 2009
|
|
T. atroviride
|
Arabidopsis
thaliana
|
Produce 6-pentyl-2H-pyran-2-one (6-PP), which promoted plant growth and regulated root architecture, inhibiting primary root growth and inducing lateral root formation.
|
Garnica-Vergara et al., 2016
|
|
T. atroviride
|
Arabidopsis
thaliana
|
Produce ethylene and improved tolerance to biotic as well as
abiotic stresses
|
Mukherjee et al., 2013
|
|
T. harzianum
|
Tomato (Solanum
lycopersicum)
|
Produce harzianolide and increase germination of tomato seeds and improved the growth of the seedlings and root development
|
Vinale et al., 2013
Cai et al., 2013
|
|
T. harzianum
|
Pea (Pisum
sativum)
|
Increase the number of lateral root and root length
|
Naseby et al., 2000
|
|
T. harzianum
|
Cucumber
(Cucumis sativus)
|
Increase in cumulative root length, root surface area, and the number of root tips
|
Yedidia et al., 2001
|
|
T. harzianum
|
Brassica (Brassica rapa) and lettuce (Lactuca sativa)
|
Produce indole-3-acetic acid (IAA) and enhance P solubilization and nutrient mineralization
|
Asghar and Kataoka, 2021
|
|
T. atroviride
|
Tomato (Solanum lycopersicum)
|
Release volatile compounds such as 2-heptanone, 2-pentyl furan (2- PF) and 6-pentyl- 2H-pyran-2-one (6- PP), promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings
|
Rao et al., 2022
|
Stress tolerance
Drought is one of the principal abiotic stresses that occurs due to water shortage and is exacerbated by rising evapotranspiration (Abdullah et al., 2021). Drought stress causes a significant reduction in the growth and yield of several important crops. Trichoderma inoculation triggers several distinct drought responses in plants (Shukla et al., 2012). For example, T. harzianum was found to postpone or delay the response of rice to drought. This was due to the promotion of root growth independent of water deficit, as evidenced by a delayed increase in the stress-induced metabolites proline, malondialdehyde (MDA) and hydrogen peroxide, as well as an increased concentration of phenolic compounds (Shukla et al., 2012). Inoculation of T. atroviride into maize plants could reduce the deleterious effects of drought and have a function in mediating resistance to stress by stimulating the antioxidant machinery that helps to overcome the unfavorable conditions caused by the overproduction of ROS (Guler et al., 2018). The T. harzianum-inoculated maize plants were shown to have high levels of starch in their leaves, which may be advantageous during drought situations when carbon deprivation is caused by extended stomatal conductance (Akladious and Abbas, 2012).
Apart from drought, cold stress poses a significant risk to the sustainability of crop yields and can result in significant crop losses (Heidarvand and Maali Amiri, 2010). Low temperatures, such as those brought on by unexpected fall frosts, winter freezing temperatures, and late spring cold episodes, can produce this stress in plants (Heidarvand and Maali Amiri, 2010). Trichoderma can suppress the reduction in plant growth and yield caused by cold stress. For instance, it was found that T. harzianum colonization mitigated the negative consequences of cold stress on the majority of commercial tomato cultivars, which are susceptible to cold (Ghorbanpour et al., 2018). T. harzianum inoculation increased the fresh and dry weights of tomato roots and leaves when compared to plants that were cold-treated. Apart from that, there was a decrease in cold injury markers like lipid peroxidation rate and electrolyte leakage and an improvement in photosynthesis and growth rate, leaf water content, and proline accumulation (Ghorbanpour et al., 2018).
Another factor restricting plant growth is soil salinity stress, which is accompanied by high osmotic potential and specific ion toxicity (Rawat et al., 2011). However, the harshness of the saline conditions was lessened when wheat plants were treated with T. harzianum (Rawat et al., 2011). Seed germination was markedly enhanced in both cucumber and Arabidopsis plants when T. asperelloides was inoculated before salt stress was imposed (Brotman et al., 2013). The supply of carbohydrates required for plant growth can be diminished by increased salt stress since it can slow down the photosynthetic rate (Ahmad et al., 2015). The photosynthetic pigment of the Indian mustard plant grown under saline conditions was significantly restored after being inoculated with T. harzianum (Ahmad et al., 2015). With T. asperellum inoculation, genes related to ROS metabolism and plant defense response were found to be up-regulated (Doni et al., 2019). Table 3 lists the alleviation of abiotic stress responses of plants after inoculation with Trichoderma spp.
Table 3. The alleviation of abiotic stress responses of plants following inoculation with Trichoderma spp.
|
Abiotic stress
|
Trichoderma spp.
|
Plants
|
Stress alleviation mechanism
|
References
|
|
Drought
|
T. harzianum
|
Rice (Oryza sativa)
|
Postpone or delay the response of rice to drought by delaying the release of stress-induced metabolites proline, malondialdehyde (MDA), and hydrogen peroxide, as well as by increasing the concentration of phenolic compounds
|
Shukla et al., 2012
|
|
T. atroviride
|
Maize
|
Stimulated the antioxidant machinery that helps to overcome drought stress by the overproduction of ROS
|
Guler et al., 2018
|
|
T. harzianum
|
Maize
|
Produced high levels of starch in leaves, which may be advantageous during droughts when extended stomatal conductance cause carbon deprivation
|
Akladious and Abbas, 2012
|
|
T. harzianum
|
Tomato
|
Increased secondary metabolites and proline content
|
Mona et al., 2017
|
|
Cold
|
T. harzianum
|
Tomato
|
Decreased cold injury markers, such as lipid peroxidation and electrolyte leakage, and an improvement in photosynthesis and growth rate, leaf water content, and proline accumulation
|
Ghorbanpour et al., 2018
|
|
Salinity
|
T. asperelloides
|
Arabidopsis,
Cucumber
|
Improved seed germination
|
Brotman et al., 2013
|
|
T. harzianum
|
Indian mustard
|
Restored photosynthetic pigment level
|
Ahmad et al., 2015
|
|
T. asperellum
|
Rice
|
Up-regulation of genes related to ROS metabolism
|
Doni et al., 2019
|
Bioremediation
Bioremediation with Trichoderma in agriculture is an excellent natural method to maintain soil fertility and increase crop yields (Zin and Badaluddin, 2020). The ability of Trichoderma to metabolize various pesticides has been demonstrated. Herbicides containing sulfonylurea are commonly used in agriculture to suppress weed growth (Vazquez et al. 2015). However, sulfonylurea kills beneficial soil microorganisms due to its non-targeted effects. Fortunately, Trichoderma has the ability to degrade sulfonylurea herbicides (Zin and Badaluddin, 2020). For example, Vazquez et al. (2015) found that T. harzianum can detoxify metsulfuron-methyl, a sulfonylurea herbicide. Trichoderma uses sulfosulphuron as a carbon source and detoxifies it by breaking down the sulfonylamide bond and the sulfonylurea bridge (Yadav and Choudhury, 2014).
Insecticides such as dichlorvos (DDVP) are frequently used in agriculture, with excessive residues in the soil endangering ecosystems and human health (Sun et al., 2019). A protein encoding a TaPon1-like protein is present in T. atroviride strain T23 and contributes to the effective biodegradation activity of DDVP (Sun et al., 2019). Carbendazim is a systemic fungicide commonly used to control soilborne diseases caused by a variety of phytopathogenic fungi (Sharma et al. 2016). However, it has been discovered to be a significant pollutant in agricultural land. Sharma et al. (2016) reported that T. harzianum, T. viride and T. amurensis could effectively degrade carbendazim within 5 days of application. Another fungicide, penthiopyrad, which is used to control foliar and soil fungal diseases in fruit, nut and vegetable crops, was also effectively degraded by T. harzianum (Linhart et al., 2019).
Commonly used pesticides are synthetic polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene, pyrene and phenanthrene. However, PAHs are among the most critical environmental pollutants due to their toxic, immobile and bioaccumulative properties (Zin and Badaluddin, 2020). In PAH-contaminated soil, T. asperellum H15 has been shown to efficiently degrade benzo[a]pyrene, pyrene, and phenanthrene by up to 81%, 63%, and 74%, respectively (Zafra et al., 2015). There is evidence that catechol 1,2 dioxygenase, laccase, and peroxidase enzymes play a key role in the degradation of PAHs by T. asperellum (Zafra et al. 2015).
Studies have shown that T. harzianum can effectively degrade a variety of harmful organic pollutants, including phenols, cyanides and nitrates (Huang et al., 2018). Trichoderma species have been shown to degrade several artificial dyes, including pentachlorophenol, endosulfan, and dichlorodiphenyl trichloroethane (DDT) (Katayama and Matsumura, 1991). In the bioremediation of Cr (VI)-contaminated wastewaters, T. inhamatum consistently reduced Cr (VI) levels to a significant extent (Morales-Barrera and Cristiani-Urbina, 2008). Similarly, T. harzianum has been reported to be effective in remediating cadmium-contaminated soils (Faedda et al., 2012). The different species of Trichoderma as bioremediators for various pollutants are presented in Table 4.
Table 4. Bioremediation of pollutants by different species of Trichoderma
|
Pollutants
|
Trichoderma spp.
|
Effects
|
References
|
|
Metsulfuron methyl (Herbicide)
|
T. harzianum
|
Detoxification of the herbicide up to 100%
|
Vázquez et al., 2015
|
|
Dichlorvos (Insecticide)
|
T. atroviride
|
Efficient degradation of the insecticide
|
Sun et al., 2019
|
|
Carbendazim (Fungicide)
|
T. harzianum
|
85% of degradation within 5 days
|
Sharma et al., 2016
|
|
|
T. atroviride
|
50% of degradation within 5 days
|
|
|
|
T. viride,
|
20% of degradation within 5 days
|
|
|
Penthiopyrad (Fungicide)
|
T. harzianum
|
60% of degradation within 14 days
|
Linhart et al., 2019
|
|
Benzo[a]pyrene
|
T. reesei
|
54% degradation after 12 days of incubation
|
Yao et al. (2015)
|
|
Benzo[a]pyrene
|
T. asperellum
|
81%, degradation in soils
|
Zafra et al., 2015
|
|
Pyrene
|
T. asperellum
|
63%, degradation in soils
|
Zafra et al., 2015
|
|
Phenanthrene
|
T. asperellum
|
74%, degradation in soils
|
Zafra et al., 2015
|
|
Carbendazim and Mancozeb
|
Trichoderma spp.
|
25% degradation of Carbendazim and 36% degradation of Mancozeb during 15 days of incubation
|
Ahlawat et al., 2010
|
|
Lead (Pb), Chromium
(Cr)
|
T. viride
|
Uptake of 9.14 mg/g of lead and 2.55 mg/g of Chromium
|
Kumar et al., 2023
|
|
Copper (Cu)
|
T. atroviride
|
50-85% of Cu adsorption during in vitro experiment
|
Yazdani et al., 2009
|
|
Zinc (Zn)
|
T. atroviride
|
47.6 – 64% adsorption and 30.4 – 45% absorption of Zn
|
Yazdani et al., 2010
|
|
Cadmium (Cd)
|
T. asperellum
|
76.17% removal of Cadmium
|
Mohsenzadeh and
Shahrokhi, 2014
|
Production of Trichoderma biofertilizer from agro-waste
There are multiple stages involved in industrial production of Trichoderma, including harvesting, drying, formulation, and storage. These steps can have a significant impact on the number of microorganisms and their viability, and consequently on the shelf life and bioefficacy of the end product (Kumar et al., 2023). The nutrient-rich, low-cost, and readily available organic substrates are needed to sustain a robust fungal growth. Agro-industrial waste possesses these properties and can be used for the large-scale production of the fungal biomass.
Several reports describe the production of Trichoderma biomass containing synthetic media such as cellulose, glucose, molasses, and starch (Kumar et al., 2023). However, the high cost of these raw materials for the commercially viable production of biocontrol agents is one of the main reasons for their limited application. For the mass production of Trichoderma, numerous studies have used low-cost substrates such as composted coir and coffee waste (Saratale et al., 2020; Van Gerrewey et al., 2020), poultry manure and coffee waste (Sawant and Sawant, 1996), neem cake, degraded coffee pulp, cow dung, and coir pith (Saju et al., 2002), sorghum grain floor, sawdust, wheat bran, groundnut shell, molasses, farm-produced waste, biogas plant extract, dried cow dung, neem cake, talc, mushroom compost, fly ash, peat soil (Sangle and Bambawale, 2005), fruit, vegetables, and crop wastes, poultry manure and farm-yard manure (FYM) (Simon, 2011), rotten wheat grains, sugarcane bagasse, fruit juice waste, and vegetable and fruit wastes (Babu and Pallavi, 2013). For large-scale production of Trichoderma, a variety of easily accessible and reasonably priced local organic media, including neem cake, coconut husk, and decomposed coffee pulp, have been suggested (Saju et al., 2002). Organic fertilizers like vermiculite, neem cake, and mushroom composts were found to be effective carriers for mass production of Trichoderma (Mustafa et al., 2009). Neem cake enriched with Trichoderma talcum powder is suggested for the treatment of coconut basal stem rot and areca nut and coconut stem bleeding (Mustafa et al., 2009). Due to the scarcity of premium neem cakes and their high cost in commercial formulations, farmers are now in need of viable biological control agents with affordable formulations for disease prevention (Kumar et al., 2023). In recent years various agro-wastes have been successfully tested for pilot-scale production of Trichoderma (Table 5).
Table 5. Different agro-waste carrier materials and their efficacy in Trichoderma biomass production
|
Agro-waste carrier materials
|
Composition/Components
|
Efficacy
|
References
|
|
Cow dung
|
Decomposed cow dung + Trichoderma formulation
|
37.5 × 107 cfu/g
|
Mohiddin et al., 2017
|
|
Coffee husk + Cow dung
|
Coffee fruit skin decomposed with cow dung + poultry manure + T. harzianum suspension
|
9 × 1011 to 3 × 1012
cfu/g substrate
|
Sawant and Sawant, 1996
|
|
Banana waste
|
Banana waste (chopped 5–6 cm length) + rock phosphate+ T. harzianum supension
|
|
Thangavelu et al., 2004
|
|
Wheat seeds
|
Grinded grain + sugar solution (1%) + T. harzianum
|
38 × 107 cfu/g
|
Mohiddin et al., 2017
|
|
Vermiculite + Oat +
bentonite
|
Oat (20 g) + bentonite (50 mL) + vermiculite + T.
harzianum + water (60 mL)
|
Maintained cfu after
8 weeks
|
Martínez-Medina et al., 2009
|
|
Rice straw
|
Fifty grams of soil + rice straw (5 g) + Trichoderma
biomass (500 mg)
|
5.3 × 1010 cfu/g
|
Organo et al., 2022
|
|
Rice powder
|
Sterilized rice powder + dextrose + talc powder +
T. viride
|
cfu/g 10 × 109 up to
six months at room
temperature
|
Rini et al., 2018
|
|
Molasses
|
Molasses yeast extract (MYE) medium+ glycerol (3%) (V/V) +T. harzianum + Talc powder
|
Extended the
shelf-life for 7 to
12 months
|
Sriram et al., 2011
|
Isolation and identification of specific Trichoderma species are essential steps that should be performed before biomass production. After isolation and characterization of a pure culture of Trichoderma, hyphae, chlamydospores or conidia can be used as propagation material (propagules). Both solid-state fermentation and submerged (liquid) fermentation can be used for the mass production of Trichoderma. Since solid-state fermentation can promote the formation of more spores, using agricultural waste to produce inoculants would be a better choice for solid-state fermentation (Kumar et al., 2023). The following processes can be used for the industrial production of Trichoderma: (1) optimization of culture conditions in the laboratory to achieve high yield and biomass; (2) optimization of biomass production at the pilot plant level to identify and solve different technical variables; (3) integration of specific unit operations from fermentation, bioseparation and formulation into a single process; and (4) production of Trichoderma on an industrial plant scale. The final product can then be tested for suitability for field applications (Abdullah et al., 2021). To improve the shelf-life and effectiveness of Trichoderma formulations, attempts have been made to create efficient and successful techniques, such as microencapsulation using various polymers, adjuvants, or carriers (Kumar et al., 2023).
Utilizing agricultural waste for biofertilizer production has its challenges, including contamination from heavy metals, pesticides, pathogens, and salts, as well as nutrient imbalances (Bhatia and Sindhu, 2024). There is also a risk of plant damage, such as smothering or scorching, an increased likelihood of disease transmission, and environmental pollution due to improper disposal methods like burning or leaching (Lackner and Besharati, 2025). To address these issues and safeguard soil and crop health, thorough evaluation and pre-treatment are essential. Certain agricultural waste can introduce toxic heavy metals, including lead, arsenic, and mercury, along with harmful chemicals into the soil, which can endanger humans, animals, and plants (Bhatia and Sindhu, 2024). Additionally, waste may carry pesticide residues that negatively affect beneficial soil microorganisms and can infiltrate the food chain. Animal manure and similar waste can contain bacteria, viruses, and parasites that pose health risks to livestock, humans, and other environmental elements (Bhatia and Sindhu, 2024). Although beneficial, the excessive use of certain waste materials, such as manure, can result in nutrient imbalances, leading to high potassium or magnesium levels that may adversely affect livestock grazing on treated pastures, potentially causing conditions like hypomagnesaemia (Lackner and Besharati, 2025). Some waste materials decompose at a slow rate, which can initially bind nutrients and impede plant growth (Lackner and Besharati, 2025). The management of biofertilizer derived from agricultural waste requires careful consideration of the following factors: (1) Pre-treatment: Waste materials typically need treatment, such as composting or biosorption, to eliminate contaminants and prepare them for fertilization, (2) Proper Application: It is vital to manage application rates and methods meticulously to prevent nutrient imbalances, plant damage, and disease spread, and (3) Source Assessment: Understanding the origin of the waste and evaluating it for potential contaminants is crucial before its use as a carrier material for biofertilizer production.
CONCLUSIONS
Trichoderma provides the following attributes for sustainable agriculture: (1) direct biocontrol of plant pests and diseases, which removes the need for chemical pesticides; (2) broad-ranging biocontrol activities against insects, nematodes, and pathogenic microorganisms; (3) activation of plant defense mechanisms, which provides indirect biocontrol of plant pests and diseases; (4) activation of plant defense mechanisms, which increases tolerance to abiotic stress; (5) stimulation of plant growth, which increases crop yields and productivity; (6) improvement of soil nutrient availability, which increases plant uptake and assimilation; and (7) stimulation of bioremediation of pesticides, organic pollutants, and heavy metals in arable soils. To optimize the benefits of this green fungus and promote safer, environmentally sustainable agriculture, multidisciplinary research is required to comprehend its multifunctional characteristics. Efforts to elucidate the molecular basis of plant growth promotion and defense activation by Trichoderma are needed to gain a broad perspective on their functions and suitability for climate-resilient agriculture. More research is needed to find more affordable alternative substrates (e.g., agricultural waste) and optimal operating conditions to increase the yield of Trichoderma biomass.
REFERENCES
Abdullah, N.S., Doni, F., Mispan, M.S., Saiman, M.Z., Yusuf, Y.M., Oke, M.A. and Suhaimi, N.S.M., 2021. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 11: 2559
Ahlawat, O.P., Gupta, P., Kumar, S., Sharma, D.K. and Ahlawat, K., 2010. Bioremediation of fungicides by spent mushroom substrate and its associated microflora. Indian J. Microbiol. 50: 390-395.
Ahmad, P., Hashem, A., Abd-Allah, E.F., Alqarawi, A.A., John, R., Egamberdieva, D., and Gucel, S., 2015. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 6: 868.
Ahmed, A.S., Ezziyyani, M., Sánchez, C.P., and Candela, M.E., 2003. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur. J. Plant Pathol. 109: 633–637.
Akladious, S.A., and Abbas, S.M., 2012. Application of Trichoderma harzianum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 11: 8672–8683.
Amin, F., and Razdan, V.K., 2010. Potential of Trichoderma species as biocontrol agents of soil borne fungal propagules. J. Phytol. 2: 38–41.
Asad, S.A., Ali, N., Hameed, A., Khan, S.A., Ahmad, R., Bilal, M., Shahzad, M. and Tabassum, A., 2014. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol. J. Microbiol. 63: 95-103.
Asghar, W., and Kataoka, R., 2021. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 203: 4281–4291.
Babu, K.N. and Pallavi, P., 2013. Isolation, identification and mass multiplication of Trichoderma an important bio-control agent. Int. J. Pharm. Life Sci. 4: 2320–2323.
Begum, M.F., Rahman, M.A., and Alam, M.F., 2010. Biological control of Alternaria fruit rot of chili by Trichoderma species under field conditions. Myco. 38: 113–117.
Bernal-Vicente, A., Ros, M., Pascual, J.A., 2009. Increased effectiveness of the Trichoderma harzianum isolate T-78 against Fusarium wilt on melon plants under nursery conditions. J. Sci. Food Agric. 89: 827–833.
Bhatia, T. and Sindhu, S.S., 2024. Sustainable management of organic agricultural wastes: contributions in nutrients availability, pollution mitigation and crop production. Discover Agriculture 2:130.
Brotman, Y., Landau, U., Cuadros-Inostroza, Á., Takayuki, T., Fernie, A.R., Chet, I., Viterbo, A., and Willmitzer, L. 2013. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 9: e1003221.
Cai, F., Yu, G.,Wang, P.,Wei, Z., Fu, L., Shen, Q., and Chen, W., 2013. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Bioch. 73: 106–113.
Contreras-Cornejo, H.A., Macías-Rodríguez, L., Alfaro-Cuevas, R., and López-Bucio, J., 2014. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe. Interact. 27: 503–514.
Contreras-Cornejo, H.A., Macías-Rodríguez, L., Cortés-Penagos, C., and López-Bucio, J., 2009. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 149: 1579–1592.
Das, S. and Kim, P.J., 2024. Biofertilizers from Agro-wastes: A Path towards Sustainable Agriculture. APBB: 1-11
Doni, F., Fathurrahman, F., Mispan, M.S., Suhaimi, N.S., Yusoff, W.M., Uphoff, N., 2019. Transcriptomic profiling of rice seedlings inoculated with the symbiotic fungus Trichoderma asperellum SL2. J. Plant Growth Regul. 38: 1507–1515.
Dunlop, R.W., Simon, A., Sivasithamparam, K., and Ghisalberti, E.L., 1989. An Antibiotic from Trichoderma Koningii active soil borne plant pathogens. J. Nat. Prod. 52: 67–74
El Komy, M.H., Saleh, A.A., Eranthodi, A., and Molan, Y.Y., 2015. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 31: 50–60.
Faedda, R., Puglisi, I., Sanzaro, V., Petrone, G. and Cacciola, S.O., 2012. Expression of genes of Trichoderma harzianum in response to the presence of cadmium in the substrate. Nat. Prod. Res. 26: 2301–2308.
Fontenelle, A., Guzzo, S., Lucon, C., and Harakava, R., 2011. Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Prot.30: 1492–1500.
Fravel, D.R., 2005. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 43: 337-359.
Galletti, S., Burzi, P.L., Cerato, C., Marinello, S. and Sala, E., 2008. Trichoderma as a potential biocontrol agent for Cercospora leaf spot of sugar beet. BioControl, 53: 917-930.
Garnica‐Vergara, A., Barrera‐Ortiz, S., Muñoz‐Parra, E., Raya‐González, J., Méndez‐Bravo, A., Macías‐Rodríguez, L., Ruiz‐Herrera, L.F. and López‐Bucio, J., 2016. The volatile 6‐pentyl‐2H‐pyran‐2‐one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytologist, 209: 1496-1512.
Ghorbanpour, A., Salimi, A., Ghanbary, M.A.T., Pirdashti, H., and Dehestani, A., 2018. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 230: 134–141.
Guler, N.S., Pehlivan, N., Karaoglu, S.A., Guzel, S., and Bozdeveci, A., 2016. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol. Planta. 38: 132.
Guzmán-Guzmán, P., Kumar, A., de Los Santos-Villalobos, S., Parra-Cota, F.I., Orozco-Mosqueda, M.D.C., Fadiji, A.E., Hyder, S., Babalola, O.O. and Santoyo, G., 2023. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 12: 432.
Harman, G.E., Howell, C.R., Viterbo, A., Chet, I. and Lorito, M., 2004. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2: 3-56.
Heidarvand, L., and Maali Amiri, R., 2010. What happens in plant molecular responses to cold stress? Acta Physiol. Plant. 32: 419–431.
Howell, C.R. 2002. Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathol. 92: 177–180.
Howell, C.R. 2006. Understanding the mechanisms employed by Trichoderma virens to effect biological control of cotton diseases. Phytopathol. 96: 178–180.
Huang, Y., Xiao, L., Li, F., Xiao, M., Lin, D., Long, X. and Wu, Z., 2018. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules, 23: 2313.
John, R.P., Tyagi, R.D., Prévost, D., Brar, S.K., Pouleur, S., and Surampalli, R.Y., 2010. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. J. Crop Prot. 29: 1452–1459.
Kashyap, P.L., Rai, P., Srivastava, A.K. and Kumar, S., 2017. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 33: 1-18.
Katayama, A. and Matsumura, F., 1991. Photochemically enhanced microbial degradation of environmental pollutants. Environ. Sci. Technol. 25: 1329–1333.
Khan, M.Y., Haque, M.M., Molla, A.H., Rahman, M.M. and Alam, M.Z., 2017. Antioxidant compounds and minerals in tomatoes by Trichoderma-enriched biofertilizer and their relationship with the soil environments. J. Integr. Agric. 16: 691-703.
Konappa, N., Krishnamurthy, S., Siddaiah, C.N., Ramachandrappa, N.S., and Chowdappa, S., 2018. Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt. J. Biol. Pest 28: 63.
Kubiak, A., Pilarska, A.A., Wolna-Maruwka, A., Niewiadomska, A. and Panasiewicz, K., 2023. The use of fungi of the Trichoderma genus in anaerobic digestion: a review. Int. J. Mol. Sci. 24: 17576.
Kumar, V., Koul, B., Taak, P., Yadav, D. and Song, M., 2023. Journey of Trichoderma from pilot scale to mass production: A review. Agriculture 13: 2022.
Lackner, M. and Besharati, M., 2025. Agricultural Waste: Challenges and Solutions, a Review. Waste 3: 18.
Lewis, J.A., and Papavizas, G.C., 1987. Application of Trichoderma and Gliocladium in alginate pellets for control of Rhizoctonia damping-off. Plant Pathol. 36: 438–446.
Li, G.Q., Huang, H.C., Acharya, S.N., and Erickson, R.S., 2005. Effectiveness of Coniothyrium minitans and Trichoderma atroviride in suppression of sclerotinia blossom blight of alfalfa. Plant Pathol. 54: 204–211.
Li, Y., Sun, R., Yu, J., Saravanakumar, K., and Chen, J. 2016. Antagonistic and biocontrol potential of Trichoderma asperellum zjsx5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 56: 318–327
Linhart, C., Niedrist, G.H., Nagler,M., Nagrani, R., Temml, V., Bardelli, T.,Wilhalm, T., Riedl, A., Zaller, J.G., Clausing, P., and Hertoge, K., 2019. Pesticide contamination and associated risk factors at public playgrounds near intensively managed apple and wine orchards. Environ. Sci. Eur. 31: 28.
Manganiello, G., Sacco, A., Ercolano, M.R., Vinale, F., Lanzuise, S., Pascale, A., Napolitano, M., Lombardi, N., Lorito, M. and Woo, S.L., 2018. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Fronti. Microbiol. 9: 1966.
Martínez-Medina, A., Roldán, and A., Pascual, J.A., 2009. Performance of a Trichoderma harzianum bentonite–vermiculite formulation against Fusarium wilt in seedling nursery melon plants. Hort. Sci. 44: 2025–2027.
Matarese, F., Sarrocco, S., Gruber, S., Seidl-Seiboth, V., and Vannacci, G., 2012. Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiol. 158: 98–106.
Metcalf, D.A. and Wilson, C.R., 2001. The process of antagonism of Sclerotium cepivorum in white rot affected onion roots by Trichoderma koningii. Plant Pathol. 50: 249–257.
Mohiddin, F., Bashir, I., Padder, S.A. and Hamid, B., 2017. Evaluation of different substrates for mass multiplication of Trichoderma species. J. Pharmacogn. Phytochem. 6: 563–569.
Mohsenzadeh, F. and Shahrokhi, F., 2014. Biological removing of Cadmium from contaminated media by fungal biomass of Trichoderma species. J. Environ. Health Sci. Engi. 12: 1-7.
Mona, S.A., Hashem, A., Abd_Allah, E.F., Alqarawi, A.A., Soliman, D.W., Wirth, S., and Egamberdieva, D., 2017. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 16: 1751–1757.
Morales-Barrera, L. and Cristiani-Urbina, E., 2008. Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water Air Soil Pollut. 187: 327–336.
Mukherjee, P.K., Horwitz, B.A., Herrera-Estrella, A., Schmoll, M., and Kenerley, C.M., 2013. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 51: 105–129.
Mustafa, A., Khan, M.A., Inam-ul-Haq, M., Khan, S., and Pervez, M.A., 2009. Mass multiplication of Trichoderma spp. on organic substrate and their effect in management of seed borne fungi. Pak. J. Phytopathol. 21: 108–114.
Naeimi, S., Okhovvat, S.M., Javan-Nikkhah, M., Vágvölgyi, C., Khosravi, V., and Kredics, L., 2010. Biological control of Rhizoctonia solani AG1-1A, the causal agent of rice sheath blight with Trichoderma strains. Phytopathol. Mediterr. 49: 287–300.
Naseby, D.C., Pascual, J.A., and Lynch, J.M., 2000. Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J. Appl. Microbiol. 88: 161–169.
Organo, N.D., Granada, S.M.J.M., Pineda, H.G.S., Sandro, J.M., Nguyen, V.H., and Gummert, M., 2022. Assessing the potential of a Trichoderma-based compost activator to hasten the decomposition of incorporated rice straw. Sci. Rep. 2022, 12, 448.
Oszust, K., Cybulska, J. and Frąc, M., 2020. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 21: 4235.
Paulitz, T.C., Ahmad, J.S., and Baker, R., 1990. Integration of Pythium nunn and Trichoderma harzianumisolate T-95 for the biological control of Pythiumdamping-off of cucumber. Plant Soil 121: 243–250.
Ramezani, H., 2008. Biological control of root-rot of eggplant caused by Macrophomina phaseolina. Am. Eurasian J. Agric. Environ. Sci. 4: 218–220.
Rao, Y., Zeng, L., Jiang, H., Mei, L., and Wang, Y., 2022. Trichoderma atroviride LZ42 releases volatile organic compounds promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings. BMC Microbiol. 22: 1–12.
Rawat, L., Singh, Y., Shukla, N., and Kumar, J., 2011. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed bio-priming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil 347: 387–400.
Rini, C., Ramya, J., Jayakumar, G., and Shajan, V., 2018. Low cost carrier material for mass production of Trichoderma inoculants. J. Trop.Agric. 56: 63–68.
Saju, K., Anandaraj, M., and Sarma, Y., 2002. On farm production of Trichoderma harzianum using organic matter. Indian Phytopathol. 55: 277–281.
Saksirirat, W., Chareerak, P. and Bunyatrachata, W., 2009. Induced systemic resistance of bio control fungus, Trichoderma sp. against bacterial and gray leaf spot in tomatoes. Asian J. Food Agro Indus. 2: 99-104.
Sangle, U. and Bambawale, O., 2005. Evaluation of substrates for mass multiplication of Trichoderma spp. Indian J. Plant Prot. 33: 298-300.
Saratale, G.D., Bhosale, R., Shobana, S., Banu, J.R., Pugazhendhi, A., Mahmoud, E., Sirohi, R., Bhatia, S.K., Atabani, A.E., Mulone, V. and Yoon, J.J., 2020. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Biores. Technol. 314: 123800.
Saravanakumar, K., Arasu, V.S., Kathiresan, K., 2013. Effect of Trichoderma on soil phosphate solubilisation and growth improvement of Avicennia marina. Aquat. Bot. 104:101–105.
Saravanakumar, K., Fan, L., Fu, K., Yu, C., Wang, M., Xia, H., Sun, J., Li, Y. and Chen, J., 2016. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci. Rep. 6: 35543.
Sawant, I.S. and Sawant, S., 1996. A simple method for achieving high cfu of Trichoderma harzianum on organic wastes for field applications. Indian Phytopathol. 49: 185–187.
Sharma, P.R., Sharma, M., Raja, M., Singh, D.V., and Srivastava, M., 2016. Use of Trichoderma spp. in biodegradation of carbendazim. Indian J. Agric. Sci. 86: 891–894.
Shukla, N., Awasthi, R., Rawat, L., and Kumar, J., 2012. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 54: 78–88.
Simon, S., 2011. Agro-based waste products as a substrate for mass production of Trichoderma spp. J. Agric. Sci. 3: 168.
Singh, S., Dureja, P., Tanwar, R., and Singh, A., 2005. Production and antifungal activity of secondary metabolites of Trichoderma virens. Pestic. Res. J. 17: 26–29.
Singh, V., Singh, P.N., Yadav, R.L., Awasthi, S.K., Joshi, B.B., Singh, R.K., Lal, R.J. and Duttamajumder, S.K., 2010. Increasing the efficacy of Trichoderma harzianum for nutrient uptake and control of red rot in sugarcane. J. Hortic. For. 2: 66-71.
Sriram, S., Roopa, K.P., and Savitha, M.J., 2011. Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop Prot. 2011, 30, 1334–1339.
Sun, J., Yuan, X., Li, Y.,Wang, X., and Chen, J., 2019. The pathway of 2, 2-dichlorovinyl dimethyl phosphate (DDVP) degradation by Trichoderma atroviride strain T23 and characterization of a paraoxonase-like enzyme. Appl. Microbiol. Biotechnol. 103: 8947–8962.
Thangavelu, R., Palaniswami, A., and Velazhahan, R., 2004. Mass production of Trichoderma harzianum for managing Fusarium wilt of banana. Agric. Ecosyst. Environ. 103: 259–263.
Van Gerrewey, T., Ameloot, N., Navarrete, O., Vandecruys, M., Perneel, M., Boon, N. and Geelen, D., 2020. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Pro. 256: 120323.
Vázquez, M.B., Barrera, V., and Bianchinotti, V., 2015. Molecular identification of three isolates of Trichoderma harzianum isolated from agricultural soils in Argentina, and their abilities to detoxify in vitro metsulfuron methyl. Bot. 93: 793–800.
Vinale, F., Nigro, M., Sivasithamparam, K., Flematti, G., Ghisalberti, E., Ruocco, M., Varlese, R., Marra, R., Lanzuise, S., Eid, A., Woo, S.L., and Lorito, M., 2013. Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS Microbiol. Letters. 347, 123–129.
Woo, S.L., Hermosa, R., Lorito, M. and Monte, E., 2023. Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 21: 312-326.
Yadav, U., and Choudhury, 2014. Biodegradation of sulfosulphuron in agricultural soil by Trichoderma sp. Lett. Appl. Microbiol. 59: 479–486.
Yao, L., Teng, Y., Luo, Y., Christie, P., Ma, W., Liu, F., Wu, Y., Luo, Y. and Li, Z., 2015. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Trichoderma reesei FS10-C and effect of bioaugmentation on an aged PAH-contaminated soil. Bioremed. J. 19: 9-17.
Yazdani, M., Yap, C.K., Abdullah, F. and Tan, S.G., 2009. Trichoderma atroviride as a bioremediator of Cu pollution: an in vitro study. Toxicol. Environ. Chem. 91: 1305-1314.
Yazdani, M., Yap, C.K., Abdullah, F. and Tan, S.G., 2010. An in vitro study on the adsorption, absorption and uptake capacity of Zn by the bioremediator Trichoderma atroviride. Environ. Asia, 3: 53-59.
Yedidia, I., Srivastva, A.K., Kapulnik, Y., and Chet, I., 2001. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 235:235–242.
Zafra, G., Moreno-Montaño, A., Absalón, Á.E., and Cortés-Espinosa, D.V., 2015. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ. Sci. Pollut. Res. 22: 1034–1042.
Zin, N.A., and Badaluddin, N.A., 2020. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 65: 168-178.
Production of Trichoderma Biofertilizer from Agro-waste for Eco-sustainable Agriculture
DOI: https://doi.org/10.56669/MMRA3791
ABSTRACT
Trichoderma is widely used in agriculture due to its known biocontrol mechanisms. By colonizing plants, either as an endophyte or on their roots, Trichoderma has developed the ability to interact with the plants and provide a variety of benefits to its hosts. The complexity of this plant-microbe association has led to a great deal of interest in the study of Trichoderma, ranging from its ability to act as a plant growth promoter to its ability to activate plant defense mechanisms against biotic and abiotic stresses. This review discusses the ideal properties of Trichoderma. It highlights its potential in biocontrol of plant diseases and pests, enhancement of nutrient use efficiency and promotion of plant growth, stress tolerance and bioremediation of organic pollutants, pesticides and heavy metals in arable soils, which are beneficial multipurpose properties for sustainable agriculture. The effectiveness of different agricultural wastes in the production of Trichoderma biofertilizers and the industrial production procedures of Trichoderma biomass are discussed.
Keywords: Biocontrol agent; plant growth promotion; stress tolerance; bioremediation
INTRODUCTION
Intensive agricultural practices and climate change have resulted in a loss of biodiversity, a change in pest and pathogen distributions, and chemical contamination of soil, air, and water resources that negatively impact the agroecosystem as well as human health. As agricultural policies are being modified to reduce the use of synthetic chemicals, there is an increasing importance of plant-beneficial fungus Trichoderma in farm practices due to its increased use as a biological alternative to agrochemicals and intensified research linking it to sustainable agriculture (Woo et al., 2023).
Trichoderma is a genus of filamentous fungi that can feed on other fungi (mycotrophism). It is found in virtually all environments, including agricultural land, forest, mountain, grassland, desert, and fresh and marine waters (Harman et al., 2004). Moreover, they are saprophytes, capable of colonizing and degrading dead organic matter. Trichoderma species thrive in all biotopes and have a broad geographic distribution worldwide. Currently, more than 375 species of Trichoderma have been identified (Kubiak et al., 2023). Trichoderma species can reproduce asexually by producing conidia (anamorph form) or sexually by producing ascospores that develop into fruiting bodies (teleomorph form). Additionally, they produce thick-walled spores, called chlamydospores, which help them survive in harsh environmental conditions (Harman et al., 2004). Trichoderma possesses strong cellulolytic and hemicellulolytic properties that allow it to accelerate the hydrolysis of polysaccharides in the cell wall of plant debris (Kumar et al., 2023). This is one of the main characteristics that favor their key application in mass production or industrial production (Kumar et al., 2023). In addition, Trichoderma species produce lignin-modifying enzymes that partially degrade this compound, which reduces its inhibitory effect on microorganisms (Kubiak et al., 2023).
As the challenges in overcoming problems in the agricultural sector have increased significantly in recent years, sustainable strategies with biological control methods are necessary. The versatile role of Trichoderma in sustainable agriculture makes it an excellent choice (Woo et al., 2023). Research interest has therefore expanded to a comprehensive analysis of the versatile properties of Trichoderma as plant-beneficial fungi for applications and improvements in agricultural production. This research interest should be aligned with the ongoing changes in agricultural policy and management and the growing concern for future sustainability.
The most important prerequisite for the commercialization of Trichoderma as a biocontrol or biofertilizer product is its ability for mass production through the economic output of the most significant number of efficient propagules (microsclerotia, conidia, and chlamydospores) in the shortest time (Fravel, 2005; Das and Kim, 2024). The main limitation for commercialization of the end product is the increased production cost due to expensive substrate or carrier materials (Fravel, 2005). However, there has been a growing interest in utilizing agricultural waste to produce biofertilizers or biocontrol products, which can not only promote sustainable agriculture and bring economic benefits to farmers but also reduce the environmental impacts associated with waste disposal. This review highlights the diverse role of Trichoderma in environmentally sustainable agriculture as well as the effectiveness of various agro-wastes in producing Trichoderma biomass.
Multifaceted role of Trichoderma in eco-sustainable agriculture
Trichoderma species are widely used as a key component in soil amendments, soil integrators, biostimulants, bioprotectants, biofertilizers, biodegraders, and bioremediators (Woo et al., 2023). They are particularly of interest to agriculture as biocontrol agents of phytopathogens, even though their many benefits have received considerable attention (Guzman-Guzman et al., 2023). Trichoderma uses direct competition and antagonism, especially in the rhizosphere, where it alters the composition of other microbes and their interactions with them. It has developed the ability to interact with plants through colonization, either on the roots or as an endophyte, and to provide a variety of diverse benefits to its host (Woo et al., 2023). Trichoderma has attracted a lot of interest in research because of its intricate relationship with plants and microorganisms, including its potential to stimulate plant growth, prime local and systemic defenses against biotic and abiotic stresses, and trigger transcriptional memory that affects future plant responses (Woo et al., 2023). Below we have discussed the diverse role of Trichoderma in biocontrol of plant diseases and pests, enhancement of nutrient use efficiency and plant growth promotion, stress tolerance, bioremediation, and above all its ideal characteristics in eco-sustainable agriculture. Figure 1 depicts the multifaceted role of Trichoderma in ecologically sustainable agriculture.
Biocontrol of plant diseases and pests
Since the 1920s, the widespread soil-dwelling fungi Trichoderma has been known to produce antibiotics and parasitize other fungi, which allows them to function as biocontrol agents against a variety of phytopathogens (Harman et al., 2004). It was later discovered that the induction of disease resistance was their primary method of protecting plants (Harman et al., 2004; Abdullah et al., 2021). Trichoderma uses both indirect and direct methods to control plant pathogens. Direct mechanisms involve mycoparasitism and coiling, while indirect mechanisms involve challenges for nutrients and space, acquired resistance, and antibiosis (Woo et al., 2023). The type of Trichoderma strains, the pathogen that is being repelled, including its host, and the ecological context all affect how effective these mechanisms are in the biocontrol strategy (Woo et al., 2023). A variety of pathogenic microorganisms that affect plants have been documented to be controlled by Trichoderma, including bacteria (Pseudomonas, Xanthomonas and Clavibacter), fungi (Fusarium, Botrytis, Colletotrichum, Erysiphe, Magnaporthe, Sclerotinia, Verticillium, Curvularia, Colletotrichum, Alternaria, Rhizoctonia, Athelia, Armillaria, Ustilago, Puccinia), oomycetes (Pythium and Phytophthora), and at least one virulent virus (green mottle mosaic virus) (Harman et al., 2004; Woo et al., 2023). The biocontrol of different Trichoderma species against plant pathogens is shown in Table 1.
Table 1. Biocontrol of different Trichoderma species against plant pathogens
Plant Disease
Crop
Causal agent
Trichoderma spp.
Reference
Bacterial wilt
Tomato (Solanum lycopersicum)
Ralstonia solanacearum
T. asperellum
Konappa et al., 2018
Fungal wilt
Tomato
Fusarium oxysporum
T. asperellum
El Komy et al., 2015
Melon (Cucumis melo)
F. oxysporum
T. harzianum
Bernal-Vicente et al., 2009
Leaf spot
Tomato
Xanthomonas euvesicatoria
T. harzianum
T. strigosum
Fontenelle et al., 2011
Cucumber (Cucumis sativus)
Pseudomonas syringae
T. harzianum
T. strigosum
Fontenelle et al., 2011
Sugar beet (Beta
vulgaris)
Cercospora
beticola
T. hermatum
Galletti et al., 2008
Damping off
Cucumber
Pythium sp.
T. harzianum
Paulitz et al., 1990
Sugar beet (Beta vulgaris)
Rhizoctonia solani
T. harzianum
Lewis and Papavizas, 1987
Cotton (Gossyphtm hirsutum)
Rhizoctonia solani
T. hamatum
Lewis and Papavizas, 1987
Cotton
Pythium aphanidermatum
T. virens
Howell, 2002
Root rot
Soybean (Glycine max)
Pythium arrhenomanes
T. viride
John et al., 2010
Corn (Zea mays)
Fusarium oxysporum
T. viride
John et al., 2010
Bean (Phaseolus
vulgaris)
Rhizoctonia
solani
T. asperellum
Asad et al., 2014
Pepper plants (Capsicum annuum)
Rhizoctonia solani
T. harzianum
Ahmed et al., 2003
Eggplant (Solanum melongena)
Macrophomina phaseolina
T. harzianum
Ramezani, 2008
Stalk rot
Maize (Zea
mays)
Fusarium
graminearum
T. asperellum
Li et al., 2016
Fruit rot
Chili (Capsicum annuum)
Alternaria tenuis
T. harzianum
Begum et al., 2010
Tomato
Rhizoctonia solani
T. viride, T. virens, T. harzianum
Amin and Razdan, 2010
Head blight
Wheat and other small grain cereals (Triticum
aestivum)
Fusarium graminearum,
F. culmorum
T. gamsii
Matarese et al., 2012
Sheath blight
Rice (Oryza sativa)
Rhizoctonia solani
T. harzianum
Naeimi et al., 2010
Blossom blight
Alfalfa (Medicago sativa)
Sclerotinia sclerotiorum
T. atroviride
Li et al., 2005
It is relatively complex to control plant diseases caused by bacteria. The use of biocontrol agents is effective at preventing bacterial pathogens and is safer for the environment than chemical bactericides. It was shown that Trichoderma inhibited the growth and survival of Ralstonia. This Gram-negative bacterium causes disease in tomato plants, due to the secretion of lysosime, viridiofungin, and trichokonin (Yan et al., 2021). Additionally, the application of Trichoderma asperellum suppressed bacterial wilt produced by the soilborne bacterium Ralstonia solanacearum, which in turn reduced the disease incidence while simultaneously improving plant growth and yield (Konappa et al., 2018). This was accomplished by increasing the total phenolic contents in plants and inducing the highest level of defense enzyme activities, including peroxidase (POX), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL), β-1,3-glucanase (Konappa et al., 2018). Biocontrol of bacterial phytopathogens is also illustrated by the induction of resistance by Trichoderma in tomato plants against Xanthomonas euvesicatoria (the causative agent of bacterial spot) and in cucumber plants against angular leaf spot caused by Pseudomonas syringae pv. Lachrymans (Fontenelle et al., 2011). Through a variety of mechanisms, such as lignification, the synthesis of phytoalexins, pathogenesis-related proteins, and secondary metabolites with antimicrobial properties, Trichoderma can protect plants from bacterial pathogens (Kumar et al., 2023).
In addition to plant diseases caused by bacteria, fungi are also often associated with plant diseases and crop damage, resulting in significant losses in agricultural production. Trichoderma has been found to have the ability to eliminate phytopathogenic fungi through various mechanisms, such as mycoparasitism, antibiosis, competition, production of antibiotics and other antifungal compounds, and induced systemic resistance (Kumar et al., 2023).
The process by which one fungus parasitizes another fungus (the host) is called mycoparasitism, and it is one of the key mechanisms of fungal antagonists (Harman et al., 2004). In addition to the pathogen cell wall penetration and host digestion, four sequential processes such as chemotaxis, identification, attachment, and wrapping have been identified as being involved in mycoparasitism (Kumar et al., 2023). Trichoderma koningii colonized injured or infected onion root tissues as a secondary colonizer, reducing Sclerotium cepivorum infection by eliminating the hyphae, rather than invading healthy tissues (Metcalf and Wilson, 2001). Conversely, Trichoderma virens reduced the inoculum potential of several pathogenic fungal species in soil by not only parasitizing their hyphae but also penetrating and destroying some of their resting structures (Howell, 2006). The enzymatic breakdown of cell walls caused by hydrolytic enzymes (such as chitinase, 1,3-glucanase, and cellulase) produced by Trichoderma leads to the degradation of host tissues containing pathogenic organisms (Kumar et al., 2023). It was shown that treating cotton seedlings with T. virens reduced the pre-emergence of damping-off disease caused by Rhizopus oryzae (Howell, 2002).
Antibiosis is the phenomenon whereby a microbe uses secondary metabolites and low molecular weight compounds and antibiotics to prevent or inhibit another organism. Trichoderma synthesizes secondary metabolites such as pyrone, heterocyclic compounds, terpenoids, polyketides, etc. and produces specific low molecular weight compounds and antibiotics to kill plant pathogens (Kumar et al., 2023). The different species of Trichoderma produce different antibiotics; for example, T. viride produces mucortoxins A and B, mucorin, trichophyton, and mucorin; T. mucorin produces mucorin A and B; T. harzianum produces tricholongins BI and BII; T. koningii produces longibrachins and trichokonins; T. atroviride produces atroviridines A-C and neoatroviridines A-D, while other antibiotics and fungicidal compounds have been isolated from T. harzianum, T. koningii, T. aureoviride, T. virens, T. hamatum, and T. lignorum. The koninginin D produced by Trichoderma inhibits the growth of soil pathogens such as Phytophthora solani, Phytophthora middletonii, Phytophthora cinnamomi, Bipolaris sorokiniana, and Fusarium oxysporum (Dunlop et al., 1989). The viridins obtained from Trichoderma species such as T. viride, T. koningii, and T. virens inhibit the germination of spores of Colletotrichum lini, Botrytis allii, Penicillium expansum, Fusarium caeruleum, Stachybotry satra, and Aspergillus niger (Singh et al., 2005). The harzianic acid produced by T. harzianum has antimicrobial activity against Sclerotinia sclerotiorum, Rhizoctonia solani, and Pythium irregular (Manganiello et al., 2018).
Fungal pathogens can be naturally controlled by competition for nutrients (Kumar et al., 2023). Certain characteristics make Trichoderma more competitive than other microorganisms, including a faster growth rate and the ability to mobilize and utilize nutrients from soil and substrate. The saprophytic ability and inoculum potential of Trichoderma are influenced by four primary characteristics: (i) fast germination of fungal propagules and rapid hyphal growth toward nutrients, (ii) production of enzymes that interact with the carbon constituents of the host plant, (iii) secretion of growth inhibitor compounds (fungistatic and bacteriostatic), and (iv) tolerance of competing microorganisms containing fungistatic compounds (Woo et al., 2023). An important factor in the interaction between Trichoderma and plant pathogens is the competition for macro and micronutrients (Harman et al., 2004). Trichoderma has been shown to compete with plant pathogens for nutrients, primarily iron, nitrogen, and carbon (Kumar et al., 2023). Trichoderma species operate as a competitor that aids in the control of plant diseases by producing iron chelating compounds and siderophores that prevent rhizospheric bacteria from obtaining iron, ultimately resulting in the extinction of the disease (Oszust et al., 2020). Studies have also found that Trichoderma can compete with plant pathogens, including Colletotrichum sp., Botrytis sp., and Phytophthora sp., for both complex and simple carbon substrates (Oszust et al., 2020).
Trichoderma confers local or systemic disease resistance by triggering a host plant's defensive mechanism while preventing pathogens from multiplying and growing (Woo et al., 2023). There are generally two methods to achieve Trichoderma-induced plant disease resistance: one is to control the inducers or stimulants that trigger plant disease resistance responses; the other is to use the cell wall-degrading enzymes produced by Trichoderma to release oligosaccharides to cause plant disease resistance (Kumar et al., 2023). It was found that Trichoderma coated corn seeds significantly increased peroxidase activity and phenylalanine ammonia lyase activity, and they proved to be resistant to Curvularia leaf spot (Saravanakumar et al., 2016). An isolate of T. harzianum was reported to induce resistance in tomato plants to bacterial spot (Xanthomonas campestris pv. vesicatoria), reducing disease incidence by 69.32% after 14 days of inoculation (Saksirirat et al., 2009).
Enhancement of nutrient use efficiency and plant growth promotion
Trichoderma can solubilize insoluble minerals via various mechanisms including redox activity and chelating metabolites and plays an important role in soil nutrient cycling (Kashyap et al., 2017). The role of Trichoderma in solubilizing tricalcium phosphate and other phosphorus has been well studied, with results indicating improved phosphorus availability to plants (Saravanakumar et al., 2013). Enhanced availability of P and Fe has been shown with significant increases in plant biomass after Trichoderma harzianum colonized cucumber roots (Yedidia et al., 2001). When T. harzianum was applied to sugarcane, the availability of N, P, and K increased by 27, 65, and 44%, respectively (Singh et al. 2010). The application of T. harzianum together with other bioagents increased the content of N, P, K, Fe, and Mg in chickpea leaves (Kashyap et al., 2017). In comparison to the recommended doses of NPK, Trichoderma biofertilizer increased tomato growth, leaf greenness, and mineral contents (P, K, Ca, Mg, Cu, Fe, Mn, and Zn) in tomato roots. It also produced a 12.9% higher yield (Khan et al., 2016). Trichoderma seed biopriming can cut the amount of nitrogen needed by 30 to 50% for a variety of crops (Harman 2011). These studies suggest that Trichoderma biofertilizers could reduce the need for chemical fertilizers, making them a recommended approach for sustainable agriculture.
Trichoderma are excellent plant growth-promoting fungi (PGPF) as they can produce plant growth-promoting substances such as indoleacetic acid (IAA), auxin and harzianic acid (Contreras-Cornejo et al., 2014). T. virens and T. atroviride were found to produce plant hormones such as indoleacetic acid (IAA) and auxin, and when Trichoderma spp. were inoculated into an Arabidopsis plant, the root tip grew (Contreras-Cornejo et al., 2014). Yedidia et al. (2001) found that a cucumber plant inoculated with T. harzianum significantly increased root biomass and increased the concentrations of Cu, P, Fe, Zn, Mn and Na in the root. Some of the Trichoderma spp. that play an important role as PGPF are listed in Table 2.
Table 2. Inoculation effects of different Trichoderma spp. on plant growth and development
Trichoderma spp.
Plant
Effects
References
T. virens
Arabidopsis
thaliana
Produce the auxin-related compounds indole-3-acetic acid, indole-3-acetaldehyde, and indole-3-ethanol and enhance plant biomass production and lateral root development
Contreras-Cornejo et al., 2009
T. atroviride
Arabidopsis
thaliana
Produce 6-pentyl-2H-pyran-2-one (6-PP), which promoted plant growth and regulated root architecture, inhibiting primary root growth and inducing lateral root formation.
Garnica-Vergara et al., 2016
T. atroviride
Arabidopsis
thaliana
Produce ethylene and improved tolerance to biotic as well as
abiotic stresses
Mukherjee et al., 2013
T. harzianum
Tomato (Solanum
lycopersicum)
Produce harzianolide and increase germination of tomato seeds and improved the growth of the seedlings and root development
Vinale et al., 2013
Cai et al., 2013
T. harzianum
Pea (Pisum
sativum)
Increase the number of lateral root and root length
Naseby et al., 2000
T. harzianum
Cucumber
(Cucumis sativus)
Increase in cumulative root length, root surface area, and the number of root tips
Yedidia et al., 2001
T. harzianum
Brassica (Brassica rapa) and lettuce (Lactuca sativa)
Produce indole-3-acetic acid (IAA) and enhance P solubilization and nutrient mineralization
Asghar and Kataoka, 2021
T. atroviride
Tomato (Solanum lycopersicum)
Release volatile compounds such as 2-heptanone, 2-pentyl furan (2- PF) and 6-pentyl- 2H-pyran-2-one (6- PP), promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings
Rao et al., 2022
Stress tolerance
Drought is one of the principal abiotic stresses that occurs due to water shortage and is exacerbated by rising evapotranspiration (Abdullah et al., 2021). Drought stress causes a significant reduction in the growth and yield of several important crops. Trichoderma inoculation triggers several distinct drought responses in plants (Shukla et al., 2012). For example, T. harzianum was found to postpone or delay the response of rice to drought. This was due to the promotion of root growth independent of water deficit, as evidenced by a delayed increase in the stress-induced metabolites proline, malondialdehyde (MDA) and hydrogen peroxide, as well as an increased concentration of phenolic compounds (Shukla et al., 2012). Inoculation of T. atroviride into maize plants could reduce the deleterious effects of drought and have a function in mediating resistance to stress by stimulating the antioxidant machinery that helps to overcome the unfavorable conditions caused by the overproduction of ROS (Guler et al., 2018). The T. harzianum-inoculated maize plants were shown to have high levels of starch in their leaves, which may be advantageous during drought situations when carbon deprivation is caused by extended stomatal conductance (Akladious and Abbas, 2012).
Apart from drought, cold stress poses a significant risk to the sustainability of crop yields and can result in significant crop losses (Heidarvand and Maali Amiri, 2010). Low temperatures, such as those brought on by unexpected fall frosts, winter freezing temperatures, and late spring cold episodes, can produce this stress in plants (Heidarvand and Maali Amiri, 2010). Trichoderma can suppress the reduction in plant growth and yield caused by cold stress. For instance, it was found that T. harzianum colonization mitigated the negative consequences of cold stress on the majority of commercial tomato cultivars, which are susceptible to cold (Ghorbanpour et al., 2018). T. harzianum inoculation increased the fresh and dry weights of tomato roots and leaves when compared to plants that were cold-treated. Apart from that, there was a decrease in cold injury markers like lipid peroxidation rate and electrolyte leakage and an improvement in photosynthesis and growth rate, leaf water content, and proline accumulation (Ghorbanpour et al., 2018).
Another factor restricting plant growth is soil salinity stress, which is accompanied by high osmotic potential and specific ion toxicity (Rawat et al., 2011). However, the harshness of the saline conditions was lessened when wheat plants were treated with T. harzianum (Rawat et al., 2011). Seed germination was markedly enhanced in both cucumber and Arabidopsis plants when T. asperelloides was inoculated before salt stress was imposed (Brotman et al., 2013). The supply of carbohydrates required for plant growth can be diminished by increased salt stress since it can slow down the photosynthetic rate (Ahmad et al., 2015). The photosynthetic pigment of the Indian mustard plant grown under saline conditions was significantly restored after being inoculated with T. harzianum (Ahmad et al., 2015). With T. asperellum inoculation, genes related to ROS metabolism and plant defense response were found to be up-regulated (Doni et al., 2019). Table 3 lists the alleviation of abiotic stress responses of plants after inoculation with Trichoderma spp.
Table 3. The alleviation of abiotic stress responses of plants following inoculation with Trichoderma spp.
Abiotic stress
Trichoderma spp.
Plants
Stress alleviation mechanism
References
Drought
T. harzianum
Rice (Oryza sativa)
Postpone or delay the response of rice to drought by delaying the release of stress-induced metabolites proline, malondialdehyde (MDA), and hydrogen peroxide, as well as by increasing the concentration of phenolic compounds
Shukla et al., 2012
T. atroviride
Maize
Stimulated the antioxidant machinery that helps to overcome drought stress by the overproduction of ROS
Guler et al., 2018
T. harzianum
Maize
Produced high levels of starch in leaves, which may be advantageous during droughts when extended stomatal conductance cause carbon deprivation
Akladious and Abbas, 2012
T. harzianum
Tomato
Increased secondary metabolites and proline content
Mona et al., 2017
Cold
T. harzianum
Tomato
Decreased cold injury markers, such as lipid peroxidation and electrolyte leakage, and an improvement in photosynthesis and growth rate, leaf water content, and proline accumulation
Ghorbanpour et al., 2018
Salinity
T. asperelloides
Arabidopsis,
Cucumber
Improved seed germination
Brotman et al., 2013
T. harzianum
Indian mustard
Restored photosynthetic pigment level
Ahmad et al., 2015
T. asperellum
Rice
Up-regulation of genes related to ROS metabolism
Doni et al., 2019
Bioremediation
Bioremediation with Trichoderma in agriculture is an excellent natural method to maintain soil fertility and increase crop yields (Zin and Badaluddin, 2020). The ability of Trichoderma to metabolize various pesticides has been demonstrated. Herbicides containing sulfonylurea are commonly used in agriculture to suppress weed growth (Vazquez et al. 2015). However, sulfonylurea kills beneficial soil microorganisms due to its non-targeted effects. Fortunately, Trichoderma has the ability to degrade sulfonylurea herbicides (Zin and Badaluddin, 2020). For example, Vazquez et al. (2015) found that T. harzianum can detoxify metsulfuron-methyl, a sulfonylurea herbicide. Trichoderma uses sulfosulphuron as a carbon source and detoxifies it by breaking down the sulfonylamide bond and the sulfonylurea bridge (Yadav and Choudhury, 2014).
Insecticides such as dichlorvos (DDVP) are frequently used in agriculture, with excessive residues in the soil endangering ecosystems and human health (Sun et al., 2019). A protein encoding a TaPon1-like protein is present in T. atroviride strain T23 and contributes to the effective biodegradation activity of DDVP (Sun et al., 2019). Carbendazim is a systemic fungicide commonly used to control soilborne diseases caused by a variety of phytopathogenic fungi (Sharma et al. 2016). However, it has been discovered to be a significant pollutant in agricultural land. Sharma et al. (2016) reported that T. harzianum, T. viride and T. amurensis could effectively degrade carbendazim within 5 days of application. Another fungicide, penthiopyrad, which is used to control foliar and soil fungal diseases in fruit, nut and vegetable crops, was also effectively degraded by T. harzianum (Linhart et al., 2019).
Commonly used pesticides are synthetic polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene, pyrene and phenanthrene. However, PAHs are among the most critical environmental pollutants due to their toxic, immobile and bioaccumulative properties (Zin and Badaluddin, 2020). In PAH-contaminated soil, T. asperellum H15 has been shown to efficiently degrade benzo[a]pyrene, pyrene, and phenanthrene by up to 81%, 63%, and 74%, respectively (Zafra et al., 2015). There is evidence that catechol 1,2 dioxygenase, laccase, and peroxidase enzymes play a key role in the degradation of PAHs by T. asperellum (Zafra et al. 2015).
Studies have shown that T. harzianum can effectively degrade a variety of harmful organic pollutants, including phenols, cyanides and nitrates (Huang et al., 2018). Trichoderma species have been shown to degrade several artificial dyes, including pentachlorophenol, endosulfan, and dichlorodiphenyl trichloroethane (DDT) (Katayama and Matsumura, 1991). In the bioremediation of Cr (VI)-contaminated wastewaters, T. inhamatum consistently reduced Cr (VI) levels to a significant extent (Morales-Barrera and Cristiani-Urbina, 2008). Similarly, T. harzianum has been reported to be effective in remediating cadmium-contaminated soils (Faedda et al., 2012). The different species of Trichoderma as bioremediators for various pollutants are presented in Table 4.
Table 4. Bioremediation of pollutants by different species of Trichoderma
Pollutants
Trichoderma spp.
Effects
References
Metsulfuron methyl (Herbicide)
T. harzianum
Detoxification of the herbicide up to 100%
Vázquez et al., 2015
Dichlorvos (Insecticide)
T. atroviride
Efficient degradation of the insecticide
Sun et al., 2019
Carbendazim (Fungicide)
T. harzianum
85% of degradation within 5 days
Sharma et al., 2016
T. atroviride
50% of degradation within 5 days
T. viride,
20% of degradation within 5 days
Penthiopyrad (Fungicide)
T. harzianum
60% of degradation within 14 days
Linhart et al., 2019
Benzo[a]pyrene
T. reesei
54% degradation after 12 days of incubation
Yao et al. (2015)
Benzo[a]pyrene
T. asperellum
81%, degradation in soils
Zafra et al., 2015
Pyrene
T. asperellum
63%, degradation in soils
Zafra et al., 2015
Phenanthrene
T. asperellum
74%, degradation in soils
Zafra et al., 2015
Carbendazim and Mancozeb
Trichoderma spp.
25% degradation of Carbendazim and 36% degradation of Mancozeb during 15 days of incubation
Ahlawat et al., 2010
Lead (Pb), Chromium
(Cr)
T. viride
Uptake of 9.14 mg/g of lead and 2.55 mg/g of Chromium
Kumar et al., 2023
Copper (Cu)
T. atroviride
50-85% of Cu adsorption during in vitro experiment
Yazdani et al., 2009
Zinc (Zn)
T. atroviride
47.6 – 64% adsorption and 30.4 – 45% absorption of Zn
Yazdani et al., 2010
Cadmium (Cd)
T. asperellum
76.17% removal of Cadmium
Mohsenzadeh and
Shahrokhi, 2014
Production of Trichoderma biofertilizer from agro-waste
There are multiple stages involved in industrial production of Trichoderma, including harvesting, drying, formulation, and storage. These steps can have a significant impact on the number of microorganisms and their viability, and consequently on the shelf life and bioefficacy of the end product (Kumar et al., 2023). The nutrient-rich, low-cost, and readily available organic substrates are needed to sustain a robust fungal growth. Agro-industrial waste possesses these properties and can be used for the large-scale production of the fungal biomass.
Several reports describe the production of Trichoderma biomass containing synthetic media such as cellulose, glucose, molasses, and starch (Kumar et al., 2023). However, the high cost of these raw materials for the commercially viable production of biocontrol agents is one of the main reasons for their limited application. For the mass production of Trichoderma, numerous studies have used low-cost substrates such as composted coir and coffee waste (Saratale et al., 2020; Van Gerrewey et al., 2020), poultry manure and coffee waste (Sawant and Sawant, 1996), neem cake, degraded coffee pulp, cow dung, and coir pith (Saju et al., 2002), sorghum grain floor, sawdust, wheat bran, groundnut shell, molasses, farm-produced waste, biogas plant extract, dried cow dung, neem cake, talc, mushroom compost, fly ash, peat soil (Sangle and Bambawale, 2005), fruit, vegetables, and crop wastes, poultry manure and farm-yard manure (FYM) (Simon, 2011), rotten wheat grains, sugarcane bagasse, fruit juice waste, and vegetable and fruit wastes (Babu and Pallavi, 2013). For large-scale production of Trichoderma, a variety of easily accessible and reasonably priced local organic media, including neem cake, coconut husk, and decomposed coffee pulp, have been suggested (Saju et al., 2002). Organic fertilizers like vermiculite, neem cake, and mushroom composts were found to be effective carriers for mass production of Trichoderma (Mustafa et al., 2009). Neem cake enriched with Trichoderma talcum powder is suggested for the treatment of coconut basal stem rot and areca nut and coconut stem bleeding (Mustafa et al., 2009). Due to the scarcity of premium neem cakes and their high cost in commercial formulations, farmers are now in need of viable biological control agents with affordable formulations for disease prevention (Kumar et al., 2023). In recent years various agro-wastes have been successfully tested for pilot-scale production of Trichoderma (Table 5).
Table 5. Different agro-waste carrier materials and their efficacy in Trichoderma biomass production
Agro-waste carrier materials
Composition/Components
Efficacy
References
Cow dung
Decomposed cow dung + Trichoderma formulation
37.5 × 107 cfu/g
Mohiddin et al., 2017
Coffee husk + Cow dung
Coffee fruit skin decomposed with cow dung + poultry manure + T. harzianum suspension
9 × 1011 to 3 × 1012
cfu/g substrate
Sawant and Sawant, 1996
Banana waste
Banana waste (chopped 5–6 cm length) + rock phosphate+ T. harzianum supension
Thangavelu et al., 2004
Wheat seeds
Grinded grain + sugar solution (1%) + T. harzianum
38 × 107 cfu/g
Mohiddin et al., 2017
Vermiculite + Oat +
bentonite
Oat (20 g) + bentonite (50 mL) + vermiculite + T.
harzianum + water (60 mL)
Maintained cfu after
8 weeks
Martínez-Medina et al., 2009
Rice straw
Fifty grams of soil + rice straw (5 g) + Trichoderma
biomass (500 mg)
5.3 × 1010 cfu/g
Organo et al., 2022
Rice powder
Sterilized rice powder + dextrose + talc powder +
T. viride
cfu/g 10 × 109 up to
six months at room
temperature
Rini et al., 2018
Molasses
Molasses yeast extract (MYE) medium+ glycerol (3%) (V/V) +T. harzianum + Talc powder
Extended the
shelf-life for 7 to
12 months
Sriram et al., 2011
Isolation and identification of specific Trichoderma species are essential steps that should be performed before biomass production. After isolation and characterization of a pure culture of Trichoderma, hyphae, chlamydospores or conidia can be used as propagation material (propagules). Both solid-state fermentation and submerged (liquid) fermentation can be used for the mass production of Trichoderma. Since solid-state fermentation can promote the formation of more spores, using agricultural waste to produce inoculants would be a better choice for solid-state fermentation (Kumar et al., 2023). The following processes can be used for the industrial production of Trichoderma: (1) optimization of culture conditions in the laboratory to achieve high yield and biomass; (2) optimization of biomass production at the pilot plant level to identify and solve different technical variables; (3) integration of specific unit operations from fermentation, bioseparation and formulation into a single process; and (4) production of Trichoderma on an industrial plant scale. The final product can then be tested for suitability for field applications (Abdullah et al., 2021). To improve the shelf-life and effectiveness of Trichoderma formulations, attempts have been made to create efficient and successful techniques, such as microencapsulation using various polymers, adjuvants, or carriers (Kumar et al., 2023).
Utilizing agricultural waste for biofertilizer production has its challenges, including contamination from heavy metals, pesticides, pathogens, and salts, as well as nutrient imbalances (Bhatia and Sindhu, 2024). There is also a risk of plant damage, such as smothering or scorching, an increased likelihood of disease transmission, and environmental pollution due to improper disposal methods like burning or leaching (Lackner and Besharati, 2025). To address these issues and safeguard soil and crop health, thorough evaluation and pre-treatment are essential. Certain agricultural waste can introduce toxic heavy metals, including lead, arsenic, and mercury, along with harmful chemicals into the soil, which can endanger humans, animals, and plants (Bhatia and Sindhu, 2024). Additionally, waste may carry pesticide residues that negatively affect beneficial soil microorganisms and can infiltrate the food chain. Animal manure and similar waste can contain bacteria, viruses, and parasites that pose health risks to livestock, humans, and other environmental elements (Bhatia and Sindhu, 2024). Although beneficial, the excessive use of certain waste materials, such as manure, can result in nutrient imbalances, leading to high potassium or magnesium levels that may adversely affect livestock grazing on treated pastures, potentially causing conditions like hypomagnesaemia (Lackner and Besharati, 2025). Some waste materials decompose at a slow rate, which can initially bind nutrients and impede plant growth (Lackner and Besharati, 2025). The management of biofertilizer derived from agricultural waste requires careful consideration of the following factors: (1) Pre-treatment: Waste materials typically need treatment, such as composting or biosorption, to eliminate contaminants and prepare them for fertilization, (2) Proper Application: It is vital to manage application rates and methods meticulously to prevent nutrient imbalances, plant damage, and disease spread, and (3) Source Assessment: Understanding the origin of the waste and evaluating it for potential contaminants is crucial before its use as a carrier material for biofertilizer production.
CONCLUSIONS
Trichoderma provides the following attributes for sustainable agriculture: (1) direct biocontrol of plant pests and diseases, which removes the need for chemical pesticides; (2) broad-ranging biocontrol activities against insects, nematodes, and pathogenic microorganisms; (3) activation of plant defense mechanisms, which provides indirect biocontrol of plant pests and diseases; (4) activation of plant defense mechanisms, which increases tolerance to abiotic stress; (5) stimulation of plant growth, which increases crop yields and productivity; (6) improvement of soil nutrient availability, which increases plant uptake and assimilation; and (7) stimulation of bioremediation of pesticides, organic pollutants, and heavy metals in arable soils. To optimize the benefits of this green fungus and promote safer, environmentally sustainable agriculture, multidisciplinary research is required to comprehend its multifunctional characteristics. Efforts to elucidate the molecular basis of plant growth promotion and defense activation by Trichoderma are needed to gain a broad perspective on their functions and suitability for climate-resilient agriculture. More research is needed to find more affordable alternative substrates (e.g., agricultural waste) and optimal operating conditions to increase the yield of Trichoderma biomass.
REFERENCES
Abdullah, N.S., Doni, F., Mispan, M.S., Saiman, M.Z., Yusuf, Y.M., Oke, M.A. and Suhaimi, N.S.M., 2021. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 11: 2559
Ahlawat, O.P., Gupta, P., Kumar, S., Sharma, D.K. and Ahlawat, K., 2010. Bioremediation of fungicides by spent mushroom substrate and its associated microflora. Indian J. Microbiol. 50: 390-395.
Ahmad, P., Hashem, A., Abd-Allah, E.F., Alqarawi, A.A., John, R., Egamberdieva, D., and Gucel, S., 2015. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 6: 868.
Ahmed, A.S., Ezziyyani, M., Sánchez, C.P., and Candela, M.E., 2003. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur. J. Plant Pathol. 109: 633–637.
Akladious, S.A., and Abbas, S.M., 2012. Application of Trichoderma harzianum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 11: 8672–8683.
Amin, F., and Razdan, V.K., 2010. Potential of Trichoderma species as biocontrol agents of soil borne fungal propagules. J. Phytol. 2: 38–41.
Asad, S.A., Ali, N., Hameed, A., Khan, S.A., Ahmad, R., Bilal, M., Shahzad, M. and Tabassum, A., 2014. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol. J. Microbiol. 63: 95-103.
Asghar, W., and Kataoka, R., 2021. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 203: 4281–4291.
Babu, K.N. and Pallavi, P., 2013. Isolation, identification and mass multiplication of Trichoderma an important bio-control agent. Int. J. Pharm. Life Sci. 4: 2320–2323.
Begum, M.F., Rahman, M.A., and Alam, M.F., 2010. Biological control of Alternaria fruit rot of chili by Trichoderma species under field conditions. Myco. 38: 113–117.
Bernal-Vicente, A., Ros, M., Pascual, J.A., 2009. Increased effectiveness of the Trichoderma harzianum isolate T-78 against Fusarium wilt on melon plants under nursery conditions. J. Sci. Food Agric. 89: 827–833.
Bhatia, T. and Sindhu, S.S., 2024. Sustainable management of organic agricultural wastes: contributions in nutrients availability, pollution mitigation and crop production. Discover Agriculture 2:130.
Brotman, Y., Landau, U., Cuadros-Inostroza, Á., Takayuki, T., Fernie, A.R., Chet, I., Viterbo, A., and Willmitzer, L. 2013. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 9: e1003221.
Cai, F., Yu, G.,Wang, P.,Wei, Z., Fu, L., Shen, Q., and Chen, W., 2013. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Bioch. 73: 106–113.
Contreras-Cornejo, H.A., Macías-Rodríguez, L., Alfaro-Cuevas, R., and López-Bucio, J., 2014. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe. Interact. 27: 503–514.
Contreras-Cornejo, H.A., Macías-Rodríguez, L., Cortés-Penagos, C., and López-Bucio, J., 2009. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 149: 1579–1592.
Das, S. and Kim, P.J., 2024. Biofertilizers from Agro-wastes: A Path towards Sustainable Agriculture. APBB: 1-11
Doni, F., Fathurrahman, F., Mispan, M.S., Suhaimi, N.S., Yusoff, W.M., Uphoff, N., 2019. Transcriptomic profiling of rice seedlings inoculated with the symbiotic fungus Trichoderma asperellum SL2. J. Plant Growth Regul. 38: 1507–1515.
Dunlop, R.W., Simon, A., Sivasithamparam, K., and Ghisalberti, E.L., 1989. An Antibiotic from Trichoderma Koningii active soil borne plant pathogens. J. Nat. Prod. 52: 67–74
El Komy, M.H., Saleh, A.A., Eranthodi, A., and Molan, Y.Y., 2015. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 31: 50–60.
Faedda, R., Puglisi, I., Sanzaro, V., Petrone, G. and Cacciola, S.O., 2012. Expression of genes of Trichoderma harzianum in response to the presence of cadmium in the substrate. Nat. Prod. Res. 26: 2301–2308.
Fontenelle, A., Guzzo, S., Lucon, C., and Harakava, R., 2011. Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Prot.30: 1492–1500.
Fravel, D.R., 2005. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 43: 337-359.
Galletti, S., Burzi, P.L., Cerato, C., Marinello, S. and Sala, E., 2008. Trichoderma as a potential biocontrol agent for Cercospora leaf spot of sugar beet. BioControl, 53: 917-930.
Garnica‐Vergara, A., Barrera‐Ortiz, S., Muñoz‐Parra, E., Raya‐González, J., Méndez‐Bravo, A., Macías‐Rodríguez, L., Ruiz‐Herrera, L.F. and López‐Bucio, J., 2016. The volatile 6‐pentyl‐2H‐pyran‐2‐one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytologist, 209: 1496-1512.
Ghorbanpour, A., Salimi, A., Ghanbary, M.A.T., Pirdashti, H., and Dehestani, A., 2018. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 230: 134–141.
Guler, N.S., Pehlivan, N., Karaoglu, S.A., Guzel, S., and Bozdeveci, A., 2016. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol. Planta. 38: 132.
Guzmán-Guzmán, P., Kumar, A., de Los Santos-Villalobos, S., Parra-Cota, F.I., Orozco-Mosqueda, M.D.C., Fadiji, A.E., Hyder, S., Babalola, O.O. and Santoyo, G., 2023. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 12: 432.
Harman, G.E., Howell, C.R., Viterbo, A., Chet, I. and Lorito, M., 2004. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2: 3-56.
Heidarvand, L., and Maali Amiri, R., 2010. What happens in plant molecular responses to cold stress? Acta Physiol. Plant. 32: 419–431.
Howell, C.R. 2002. Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathol. 92: 177–180.
Howell, C.R. 2006. Understanding the mechanisms employed by Trichoderma virens to effect biological control of cotton diseases. Phytopathol. 96: 178–180.
Huang, Y., Xiao, L., Li, F., Xiao, M., Lin, D., Long, X. and Wu, Z., 2018. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules, 23: 2313.
John, R.P., Tyagi, R.D., Prévost, D., Brar, S.K., Pouleur, S., and Surampalli, R.Y., 2010. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. J. Crop Prot. 29: 1452–1459.
Kashyap, P.L., Rai, P., Srivastava, A.K. and Kumar, S., 2017. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 33: 1-18.
Katayama, A. and Matsumura, F., 1991. Photochemically enhanced microbial degradation of environmental pollutants. Environ. Sci. Technol. 25: 1329–1333.
Khan, M.Y., Haque, M.M., Molla, A.H., Rahman, M.M. and Alam, M.Z., 2017. Antioxidant compounds and minerals in tomatoes by Trichoderma-enriched biofertilizer and their relationship with the soil environments. J. Integr. Agric. 16: 691-703.
Konappa, N., Krishnamurthy, S., Siddaiah, C.N., Ramachandrappa, N.S., and Chowdappa, S., 2018. Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt. J. Biol. Pest 28: 63.
Kubiak, A., Pilarska, A.A., Wolna-Maruwka, A., Niewiadomska, A. and Panasiewicz, K., 2023. The use of fungi of the Trichoderma genus in anaerobic digestion: a review. Int. J. Mol. Sci. 24: 17576.
Kumar, V., Koul, B., Taak, P., Yadav, D. and Song, M., 2023. Journey of Trichoderma from pilot scale to mass production: A review. Agriculture 13: 2022.
Lackner, M. and Besharati, M., 2025. Agricultural Waste: Challenges and Solutions, a Review. Waste 3: 18.
Lewis, J.A., and Papavizas, G.C., 1987. Application of Trichoderma and Gliocladium in alginate pellets for control of Rhizoctonia damping-off. Plant Pathol. 36: 438–446.
Li, G.Q., Huang, H.C., Acharya, S.N., and Erickson, R.S., 2005. Effectiveness of Coniothyrium minitans and Trichoderma atroviride in suppression of sclerotinia blossom blight of alfalfa. Plant Pathol. 54: 204–211.
Li, Y., Sun, R., Yu, J., Saravanakumar, K., and Chen, J. 2016. Antagonistic and biocontrol potential of Trichoderma asperellum zjsx5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 56: 318–327
Linhart, C., Niedrist, G.H., Nagler,M., Nagrani, R., Temml, V., Bardelli, T.,Wilhalm, T., Riedl, A., Zaller, J.G., Clausing, P., and Hertoge, K., 2019. Pesticide contamination and associated risk factors at public playgrounds near intensively managed apple and wine orchards. Environ. Sci. Eur. 31: 28.
Manganiello, G., Sacco, A., Ercolano, M.R., Vinale, F., Lanzuise, S., Pascale, A., Napolitano, M., Lombardi, N., Lorito, M. and Woo, S.L., 2018. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Fronti. Microbiol. 9: 1966.
Martínez-Medina, A., Roldán, and A., Pascual, J.A., 2009. Performance of a Trichoderma harzianum bentonite–vermiculite formulation against Fusarium wilt in seedling nursery melon plants. Hort. Sci. 44: 2025–2027.
Matarese, F., Sarrocco, S., Gruber, S., Seidl-Seiboth, V., and Vannacci, G., 2012. Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiol. 158: 98–106.
Metcalf, D.A. and Wilson, C.R., 2001. The process of antagonism of Sclerotium cepivorum in white rot affected onion roots by Trichoderma koningii. Plant Pathol. 50: 249–257.
Mohiddin, F., Bashir, I., Padder, S.A. and Hamid, B., 2017. Evaluation of different substrates for mass multiplication of Trichoderma species. J. Pharmacogn. Phytochem. 6: 563–569.
Mohsenzadeh, F. and Shahrokhi, F., 2014. Biological removing of Cadmium from contaminated media by fungal biomass of Trichoderma species. J. Environ. Health Sci. Engi. 12: 1-7.
Mona, S.A., Hashem, A., Abd_Allah, E.F., Alqarawi, A.A., Soliman, D.W., Wirth, S., and Egamberdieva, D., 2017. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 16: 1751–1757.
Morales-Barrera, L. and Cristiani-Urbina, E., 2008. Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water Air Soil Pollut. 187: 327–336.
Mukherjee, P.K., Horwitz, B.A., Herrera-Estrella, A., Schmoll, M., and Kenerley, C.M., 2013. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 51: 105–129.
Mustafa, A., Khan, M.A., Inam-ul-Haq, M., Khan, S., and Pervez, M.A., 2009. Mass multiplication of Trichoderma spp. on organic substrate and their effect in management of seed borne fungi. Pak. J. Phytopathol. 21: 108–114.
Naeimi, S., Okhovvat, S.M., Javan-Nikkhah, M., Vágvölgyi, C., Khosravi, V., and Kredics, L., 2010. Biological control of Rhizoctonia solani AG1-1A, the causal agent of rice sheath blight with Trichoderma strains. Phytopathol. Mediterr. 49: 287–300.
Naseby, D.C., Pascual, J.A., and Lynch, J.M., 2000. Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J. Appl. Microbiol. 88: 161–169.
Organo, N.D., Granada, S.M.J.M., Pineda, H.G.S., Sandro, J.M., Nguyen, V.H., and Gummert, M., 2022. Assessing the potential of a Trichoderma-based compost activator to hasten the decomposition of incorporated rice straw. Sci. Rep. 2022, 12, 448.
Oszust, K., Cybulska, J. and Frąc, M., 2020. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 21: 4235.
Paulitz, T.C., Ahmad, J.S., and Baker, R., 1990. Integration of Pythium nunn and Trichoderma harzianumisolate T-95 for the biological control of Pythiumdamping-off of cucumber. Plant Soil 121: 243–250.
Ramezani, H., 2008. Biological control of root-rot of eggplant caused by Macrophomina phaseolina. Am. Eurasian J. Agric. Environ. Sci. 4: 218–220.
Rao, Y., Zeng, L., Jiang, H., Mei, L., and Wang, Y., 2022. Trichoderma atroviride LZ42 releases volatile organic compounds promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings. BMC Microbiol. 22: 1–12.
Rawat, L., Singh, Y., Shukla, N., and Kumar, J., 2011. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed bio-priming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil 347: 387–400.
Rini, C., Ramya, J., Jayakumar, G., and Shajan, V., 2018. Low cost carrier material for mass production of Trichoderma inoculants. J. Trop.Agric. 56: 63–68.
Saju, K., Anandaraj, M., and Sarma, Y., 2002. On farm production of Trichoderma harzianum using organic matter. Indian Phytopathol. 55: 277–281.
Saksirirat, W., Chareerak, P. and Bunyatrachata, W., 2009. Induced systemic resistance of bio control fungus, Trichoderma sp. against bacterial and gray leaf spot in tomatoes. Asian J. Food Agro Indus. 2: 99-104.
Sangle, U. and Bambawale, O., 2005. Evaluation of substrates for mass multiplication of Trichoderma spp. Indian J. Plant Prot. 33: 298-300.
Saratale, G.D., Bhosale, R., Shobana, S., Banu, J.R., Pugazhendhi, A., Mahmoud, E., Sirohi, R., Bhatia, S.K., Atabani, A.E., Mulone, V. and Yoon, J.J., 2020. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Biores. Technol. 314: 123800.
Saravanakumar, K., Arasu, V.S., Kathiresan, K., 2013. Effect of Trichoderma on soil phosphate solubilisation and growth improvement of Avicennia marina. Aquat. Bot. 104:101–105.
Saravanakumar, K., Fan, L., Fu, K., Yu, C., Wang, M., Xia, H., Sun, J., Li, Y. and Chen, J., 2016. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci. Rep. 6: 35543.
Sawant, I.S. and Sawant, S., 1996. A simple method for achieving high cfu of Trichoderma harzianum on organic wastes for field applications. Indian Phytopathol. 49: 185–187.
Sharma, P.R., Sharma, M., Raja, M., Singh, D.V., and Srivastava, M., 2016. Use of Trichoderma spp. in biodegradation of carbendazim. Indian J. Agric. Sci. 86: 891–894.
Shukla, N., Awasthi, R., Rawat, L., and Kumar, J., 2012. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 54: 78–88.
Simon, S., 2011. Agro-based waste products as a substrate for mass production of Trichoderma spp. J. Agric. Sci. 3: 168.
Singh, S., Dureja, P., Tanwar, R., and Singh, A., 2005. Production and antifungal activity of secondary metabolites of Trichoderma virens. Pestic. Res. J. 17: 26–29.
Singh, V., Singh, P.N., Yadav, R.L., Awasthi, S.K., Joshi, B.B., Singh, R.K., Lal, R.J. and Duttamajumder, S.K., 2010. Increasing the efficacy of Trichoderma harzianum for nutrient uptake and control of red rot in sugarcane. J. Hortic. For. 2: 66-71.
Sriram, S., Roopa, K.P., and Savitha, M.J., 2011. Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop Prot. 2011, 30, 1334–1339.
Sun, J., Yuan, X., Li, Y.,Wang, X., and Chen, J., 2019. The pathway of 2, 2-dichlorovinyl dimethyl phosphate (DDVP) degradation by Trichoderma atroviride strain T23 and characterization of a paraoxonase-like enzyme. Appl. Microbiol. Biotechnol. 103: 8947–8962.
Thangavelu, R., Palaniswami, A., and Velazhahan, R., 2004. Mass production of Trichoderma harzianum for managing Fusarium wilt of banana. Agric. Ecosyst. Environ. 103: 259–263.
Van Gerrewey, T., Ameloot, N., Navarrete, O., Vandecruys, M., Perneel, M., Boon, N. and Geelen, D., 2020. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Pro. 256: 120323.
Vázquez, M.B., Barrera, V., and Bianchinotti, V., 2015. Molecular identification of three isolates of Trichoderma harzianum isolated from agricultural soils in Argentina, and their abilities to detoxify in vitro metsulfuron methyl. Bot. 93: 793–800.
Vinale, F., Nigro, M., Sivasithamparam, K., Flematti, G., Ghisalberti, E., Ruocco, M., Varlese, R., Marra, R., Lanzuise, S., Eid, A., Woo, S.L., and Lorito, M., 2013. Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS Microbiol. Letters. 347, 123–129.
Woo, S.L., Hermosa, R., Lorito, M. and Monte, E., 2023. Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 21: 312-326.
Yadav, U., and Choudhury, 2014. Biodegradation of sulfosulphuron in agricultural soil by Trichoderma sp. Lett. Appl. Microbiol. 59: 479–486.
Yao, L., Teng, Y., Luo, Y., Christie, P., Ma, W., Liu, F., Wu, Y., Luo, Y. and Li, Z., 2015. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Trichoderma reesei FS10-C and effect of bioaugmentation on an aged PAH-contaminated soil. Bioremed. J. 19: 9-17.
Yazdani, M., Yap, C.K., Abdullah, F. and Tan, S.G., 2009. Trichoderma atroviride as a bioremediator of Cu pollution: an in vitro study. Toxicol. Environ. Chem. 91: 1305-1314.
Yazdani, M., Yap, C.K., Abdullah, F. and Tan, S.G., 2010. An in vitro study on the adsorption, absorption and uptake capacity of Zn by the bioremediator Trichoderma atroviride. Environ. Asia, 3: 53-59.
Yedidia, I., Srivastva, A.K., Kapulnik, Y., and Chet, I., 2001. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 235:235–242.
Zafra, G., Moreno-Montaño, A., Absalón, Á.E., and Cortés-Espinosa, D.V., 2015. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ. Sci. Pollut. Res. 22: 1034–1042.
Zin, N.A., and Badaluddin, N.A., 2020. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 65: 168-178.