DOI: https://doi.org/10.56669/YRAU4781
ABSTRACT
The European Union Fertilizing Products Regulation (EU Regulation No 2019/2009, FPR) establishes a unified and stringent legal framework governing the approval, marketing, and use of fertilizing products, including plant biostimulants (PBs). Unlike its predecessor (EC Regulation No 2003/2003), which primarily focused on chemically synthesized inorganic fertilizers and lacked comprehensive oversight, the FPR expands regulatory coverage to include fertilizers derived from recycled organic materials while ensuring safety for human health and the environment. The PFR comprises eight chapters and fifty-three articles, addressing key areas such as the obligations of economic operators, product compliance, and notification by conformity assessment bodies. For the first time globally, PBs are formally defined and categorized under Product Function Category 6 (PFC 6), specifying four primary functions. Registration of PBs under the FPR requires compliance with product function definitions, composition categories, labelling requirements, and successful completion of conformity assessment procedures. Upon approval, products must bear the CE mark and submit an EU Declaration of Conformity, allowing free circulation within the EU market. However, several compliance challenges remain, including classification disputes, composition restrictions, digital labelling complexities, and administrative burdens. The European Biostimulants Industry Council (EBIC) continues to work closely with regulatory authorities to address these issues. The EU’s regulatory experience offers valuable insights for other regions, including the Asia-Pacific, as they seek to develop science-based, harmonized regulations that balance innovation, safety, and market accessibility for plant biostimulants.
Keywords: EU Fertilizing Products Regulation, plant biostimulants, definition, registration
OVERVIEW OF REGULATION (EU) No 2019/1009
The European Parliament and the Council of the European Union published Regulation (EU) No 2019/2009 (Fertilizing Products Regulation, FPR) on June 5, 2019, repealing the previous Regulation (EC) No 2003/2003 and amending the Regulation (EC) No 1107/2009; additionally, the revised current FPR was published on June 26, 2022, and is applicable from July 16, 2022 (EU, 2019). The main regulatory framework for regulating EU fertilizer products in conjunction with the FPR is listed in Table 1.
Table 1. The main regulatory framework of Regulation (EU) No 2019/1009.
|
EU Regulation
|
Main Regulatory Scope
|
|
Regulation (EC) 1907/2006
|
Regulates the registration, evaluation, authorization, and restriction of chemical substances to ensure safety for human health and the environment.
|
|
Regulation (EU) 1025/2012
|
Rules on the establishment of European standards.
|
|
Regulation (EC) No 1272/2008
|
EU regulations on the classification, labeling, and packaging of substances and mixtures.
|
|
Regulation (EC) No 1069/2009
|
Health rules on animal by-products
|
|
Regulation (EC) No 765/2008
|
Specifies requirements for CE certification of products, national certification bodies, and market surveillance.
|
|
Regulation (EU) No 2019/1020
|
Regulates market surveillance and compliance of products.
|
* In the EU, any chemical substance manufactured or imported in quantities exceeding 1 tonne per year must have its safety ensured by the manufacturer or importer, and it may only be placed on the EU market once registration has been completed. Annex II of the Fertilizing Products Regulation (EU 2019/1009) subjects CMCs 1, 6, 8, 11–15 to stricter requirements (“REACH+”) than those under REACH (EC 1907/2006) when substances are produced at <10 t/year for use in EU fertilizing products.
Regulation (EU) No 2019/2009 consists of 8 chapters and 53 articles and Appendix I-V, including general provisions, obligations of economic operators, conformity of EU fertilizing products, notification of conformity assessment bodies, Union market surveillance, delegated powers and committee procedure, amendments and transitional and final provisions. The regulations regarding the conditions and obligations of economic operators and conformity assessment bodies are fundamental, summarized as follows:
- Obligations of manufacturers:
・ Before placing a product on the market, the manufacturer shall ensure that the product complies with the requirements of Annex I and II.
・ The manufacturer shall submit the technical documentation by the relevant conformity assessment procedures described in Article 15.
・ After successful conformity assessment, the manufacturer shall ensure that the product is accompanied by the labeling information required by Annex III.
・ Affix the CE mark on the product.
・ Preparation of an EU declaration of conformity.
・ Retain technical documentation and the EU Declaration of Conformity for 5 years after the product is placed on the market.
・ Provide copies of the EU statement of conformity to other economic operators.
- Basic conditions and obligations of the notified body:
・ EU member states shall designate a notified body as a conformity assessment body.
・ The notified body shall be established in accordance with the national laws of the member states, conducted by a national certification authority following the meanings and provisions of Regulation (EC) No 765/2008.
・ This notified body must be a third-party conformity assessment body with legal personality.
・ Requirements for personnel and subsidiaries responsible for conformity assessment tasks:
- Adequate knowledge to understand the requirements specified in Annex I, Annex II, and Annex III, the applicable coordination standards mentioned in Article 13, and the relevant provisions of the common specifications referred to in Article 14.
- Conformity assessors must have the ability to prepare inspection certificates.
- Responsible for establishing and implementing conformity assessment and notifications, as well as adhering to professional secrecy.
- The conformity assessment body must also ensure that the actions of its subsidiaries or subcontractors do not affect the confidentiality, objectivity, or impartiality of its conformity assessment.
REQUIREMENTS FOR EU FERTILIZER PRODUCTS
- Meet the requirements set out in Annex I for the relevant product function category
Appendix I is a list of 7 functional categories (PFC1-7) of EU fertilizer products, including fertilizers (organic, organo-mineral fertilizers and inorganic fertilizers), liming material, soil improver, growing medium, inhibitors, plant biostimulants (PBs) and fertilizing product blend (i.e., products consisting of two or more EU fertilizer products from PFC 1 to PFC 6) (Table 2).
Table 2. Product functionality categories of EU fertilizers.
|
PFC 1
|
Fertiliser
|
| |
A.
|
Organic fertiliser
|
| |
|
I. Solid organic fertiliser
|
| |
|
II. Liquid organic fertiliser
|
| |
B.
|
Organo-mineral fertiliser
|
| |
|
I. Solid organic fertiliser
|
| |
|
II. Liquid organic fertiliser
|
| |
C.
|
Inorganic fertiliser
|
| |
|
I. Inorganic macronutrient fertiliser
|
| |
|
II. Inorganic micronutrient fertiliser
|
|
PFC 2
|
Liming material
|
|
PFC 3
|
Soil improver
|
| |
A.
|
Organic soil improver
|
| |
B.
|
Inorganic soil improver
|
|
PFC 4
|
Growing medium
|
|
PFC 5
|
Inhibitor
|
| |
A.
|
Nitrification inhibitor
|
| |
B.
|
Denitrification inhibitor
|
| |
C.
|
Urease inhibitor
|
|
PFC 6
|
Plant biostimulant
|
| |
A.
|
Microbial plant biostimulant
|
| |
B.
|
Non-microbial plant biostimulant
|
|
PFC 7
|
Fertilising product blend
|
- Meet the requirements set out in Annex II for the relevant component material category or categories
An EU fertilizing product shall consist solely of component materials complying with the requirements for one or more of the CMCs listed in Annex II. There are 11 component material categories of the current FPR, including virgin material substances and mixtures (CMC1); plants, plant parts, or plant extracts (CMC2); compost (CMC3); fresh crop digestate (CMC4); digestate other than fresh crop digestate (CMC5); food industry by-products (CMC6); micro-organisms (CMC7); nutrient polymers (CMC8); polymers other than nutrient polymers (CMC9); Derived products within the meaning of Regulation (EC) No 1069/2009 (CMC 10) and by-products within the meaning of Directive 2008/98/EC (CMC11).
The November 20, 2024 revision of the FPR has been increased to 15 items, including precipitated phosphate salts and derivatives (CMC12), thermal oxidation materials and derivatives (CMC13), pyrolysis and gasification materials (CMC14), and recovered high-purity materials (CMC15) (Table 3). At the same time, animal-derived products (CMC10) have also been amended to allow the registration of Category 3 animal-derived substances as organic fertilizers and soil improvers if the product complies with Article 3(d) of the Delegated Regulation 2023/1605 (Supplementary Regulation (EU) No 1069/2009) to processed manure at the end of the manufacturing chain.
Table 3. Component material categories of EU fertilizers.
|
CMC 1: Virgin material substances and mixtures
|
|
CMC 2: Plants, plant parts or plant extracts
|
|
CMC 3: Compose
|
|
CMC 4: Fresh crop digestate
|
|
CMC 5: Digestate other than fresh crop digestate
|
|
CMC 6: Food industry by-products
|
|
CMC 7: micro-organisms
|
|
CMC 8: Nutrient polymers
|
|
CMC 9: Polymers other than nutrient polymers
|
|
CMC 10: Derived products within the meaning of Regulation (EC) No 1069/2009
|
|
CMC 11: By-products within the meaning of Directive 2008/98/EC
|
|
CMC 12: Precipitated phosphate salts and derivates
|
|
CMC 13: Thermal oxidation materials or derivates
|
|
CMC 14: Pyrolysis and gasification materials
|
|
CMC 15: Recovered high purity materials
|
3. Be labeled in accordance with the labeling requirements set out in Annex III
1) The product name and claimed functions as shown in Annex I.
2) the quantity of the EU fertilizing product, indicated by mass or volume.
3) Description of the expected use, including application rates, timing, frequency, and target plants.
4) Recommended storage conditions.
5) Products of CMC 9 polymers shall be in accordance with the instructions for use referred to in Part 2(d) of Annex II.
6) Any relevant information on measures recommended to manage risks to human, animal or plant health, to safety or the environment
7) A list of all ingredients exceeding 5%, arranged in descending order by dry weight; if the ingredient is a substance or mixture, it should be labeled according to Article 18 of Regulation (EC) No 1272/2008 on the classification, labeling, and packaging of substances and mixtures.
The labeling requirements for special products are based on the characteristics of different functional products, and separate specifications are formulated.
Tolerance rules
In FPR, the allowable values for the composition of different functional products are determined mainly due to possible deviations in manufacturing, sampling, and analysis of fertilizer products. The tolerances allowed in respect of the declared parameters indicated in this part include both negative and positive values.
4. Pass the conformity assessment inspection
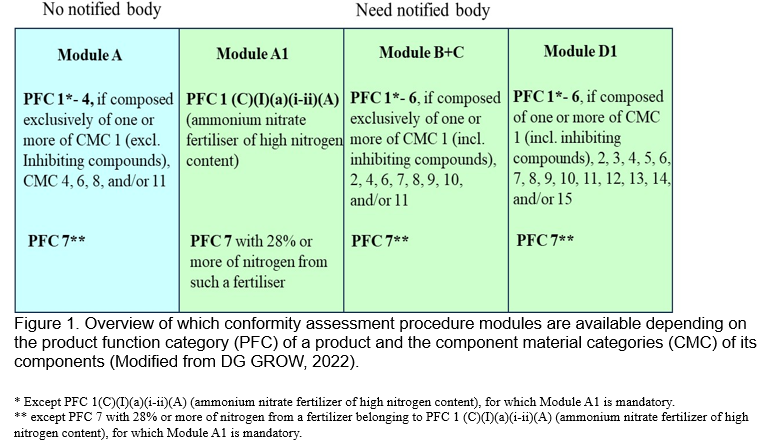
In addition to meeting the previous three requirements, registered as an EU fertilizer product must pass the conformity assessment. The conformity assessment modules applicable to different functional products mainly depend on the functional category (PFC, Annex I) and its component materials category (CMC, Annex II). There are four types of modules A, A1, B+C and D1 for conformity assessment, of which the A1, B+C and D1 modules need to be certified by a notified body. For example, module A is suitable for internal production control of CMC1, 4, 6, 7, 11 and PFC 7, and should not be used for PFCs 1(C)(I)(a)(i-ii)(A), PFC5 and PFC6. Module A1 applies to PFC 1(C)(I)(a)(i-ii)(A) and PFC 1(C)(Iii)(A) for internal production control and supervised product testing. Module B is suitable for EU-type examination and module C is ideal for type conformity review for internal production control, so module B+C is suitable for one or more constituent products of CMC1, CMC2, CMC7, CMC9, CMC10, PFC5, PFC6 and PFC7. Module D1 is a quality assurance assessment for the production process and can be applied to any EU fertilizer products (except ammonia nitrate fertilizers) (Fig. 1) (Grow, 2022; van Schöll et al., 2023).
REGULATION OF EU PLANT BIOSTIMULANTS
1. Definition of PBs
Plant biostimulants are classified under product function category 6 (PFC 6) in FPR, with the functional requirements for PBs clarified in Appendix I: ‟an EU fertilizing product the function of which is to stimulate plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency, (b) tolerance to abiotic stress, (c) quality traits, or (d) availability of confined nutrients in the soil or rhizosphere. ”
2. Rationale for attributing PBs to fertilizers
According to point 22 of the preamble of FPR: ‟plant biostimulants are not as such inputs of nutrients, but stimulate plants’ natural nutrition processes. Where such products aim solely at improving the plants’ nutrient use efficiency, tolerance to abiotic stress, quality traits or increasing the availability of confined nutrients in the soil or rhizosphere, they are by nature more like fertilizing products than to most categories of plant protection products. They act in addition to fertilizers, intending to optimise the efficiency of those fertilizers and reduce the nutrient application rates. Such products should therefore be eligible for CE marking under this Regulation and shall not fall within the scope of Regulation (EC) No 1107/2009.” As a result, the European Parliament and the Council have classified PBs in the PFC 6 category under Regulation (EU) 2019/1009.
3. Requirements for registration as an EU PBs
1) Commonly used components materials of PBs
At present, the main components of commercially available PBs include amino acids, hydrolyzed proteins, seaweed extracts, humic acid, fulvic acid, micro-organisms, chitosan, etc., which correspond to the CMC of FPR and can be covered in the categories of CMC1, CMC2, CMC6, CMC7, CMC8, and CMC11. According to Part II Requirements related to CMCs, in which CMC1, CMC6, CMC8 and CMC11, whether used alone or in a mixture, should be registered in accordance with Regulation (EC) 1907/2006 (Registration, Evaluation and Authorization of Chemical Substances, REACH). The required documentation includes the technical documentation required by Article 10 of Annex VI, information requirements from Annex VII or VIII (depending on production or import volume), and the chemical safety report stipulated in Article 14 of the regulation. The purpose of this regulation is to protect human health and the environment from the hazards of chemical substances.
As animal-based hydrolyzed protein is an animal-derived product, it is currently regulated by Regulation (EC) No 1069/2009 and has not yet been included in the CMC10 of the current FPR. Therefore, PBs with animal hydrolyzed protein as the main ingredient cannot be registered as EU PBs products at present.
To ensure that EU fertilizer commodities do not adversely affect human and animal health and environmental safety, the levels of heavy metals in PBs and pathogen levels in microbial PBs and non-microbial PBs are limited in the FPR as shown in Table 4-6.
Table 4. Contaminants in a plant biostimulants must not exceed the limit.
|
metal
|
mg kg-1 dry matter
|
|
cadmium (Cd)
|
1.5
|
|
hexavalent chromium (Cr VI)
|
2
|
|
lead (Pb)
|
120
|
|
mercury (Hg)
|
1
|
|
nickel (Ni)
|
50
|
|
inorganic arsenic (As)
|
40
|
|
copper (Cu)
|
600
|
|
zinc (Zn)
|
500
|
Table 5. Pathogens in microbial plant biostimulants must not exceed the limits.
|
Micro-organisms/their toxins, metabolites
|
Sampling plans
|
Limit
|
|
n
|
c
|
|
Salmonella spp.
|
5
|
0
|
Absence in 25 g or 25 ml
|
|
Escherichia coli
|
5
|
0
|
Absence in 1 g or 1 ml
|
|
Listeria monocytogenes
|
5
|
0
|
Absence in 25 g or 25 ml
|
|
Vibrio spp.
|
5
|
0
|
Absence in 25 g or 25 ml
|
|
Shigella spp.
|
5
|
0
|
Absence in 25 g or 25 ml
|
|
Staphylococcus aureus
|
5
|
0
|
Absence in 25 g or 25 ml
|
|
Enterococcaceae
|
5
|
2
|
10 CFU/g
|
|
Anaerobic plate count unless the microbial plant biostimulant is an aerobic bacterium
|
5
|
2
|
105 CFU/g or ml
|
|
Yeast and mould count unless the microbial plant biostimulant is a fungus
|
5
|
2
|
1 000 CFU/g or ml
|
n = number of units comprising the sample,
c = number of sample units giving values over the defined limit.
Table 6. Pathogens in a non-microbial plant biostimulants must not exceed the limits.
|
Micro-organisms to be tested
|
Sampling plans
|
Limit
|
|
n
|
c
|
m
|
M
|
|
Salmonella spp.
|
5
|
0
|
0
|
Absence in 25 g or 25 ml
|
|
Escherichia coli or Enterococcaceae
|
5
|
5
|
0
|
1 000 in 1 g or 1 ml
|
n= number of samples to be tested,
c = number of samples where the number of bacteria expressed in CFU is between m and M,
m= threshold value for the number of bacteria expressed in CFU that is considered satisfactory,
M = maximum value of the number of bacteria expressed in CFU.
2) Labeling requirements
In addition to the general labeling requirements, the label of PBs must provide information including physical form, expiration date, method of application, claimed efficacy to the target plant, and any description of the efficacy of the product, including soil management measures, chemical fertilizers, incompatibility with pesticides, recommended nozzle size, aerosol pressure, and other anti-drift measures. Microbial PBs (PFC 6 (A)) should also be labelled for all added micro-organisms at levels expressed in active units per volume or weight, or in colony forming units per gram (cfu/g), and the following warning may be included on the label: “Microorganisms may have the potential to cause sensitization reactions”.
Tolerance values of PBs: Microbial PBs (PFC 6 (A)) and Non-Microbial PBs (PFC 6 (B)) have allowable tolerances of ±15 % and ±5 %, respectively. If the fertilizer product blend (PFC 7) contains one or more PBs, the concentration of each PB is expressed in g/kg or g/l.
3) Conformity assessment of PBs is applicable to the B+C module
Follows the FPR specifications, and the qualified assessment module B+C is suitable for the testing of PBs. The conformity assessment includes testing, inspection and certification, and must pass a conformity assessment to ensure that the product meets safety, effectiveness and environmental standards, as shown in Fig. 2 (van Schöll et al., 2023).
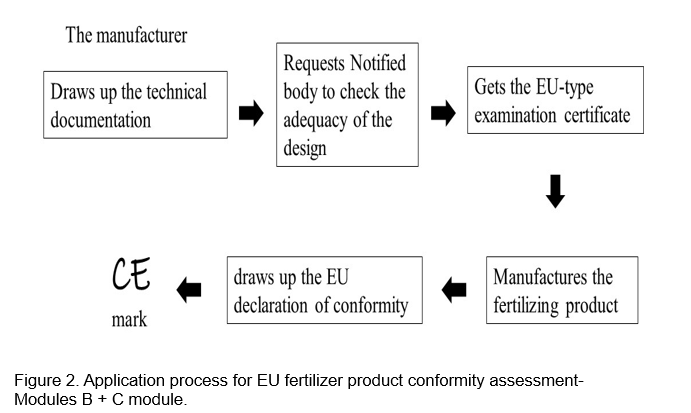
Module B is part of the EU-type examination, in which the technical design of EU fertilizer products is reviewed by a Notified Body and the technical design of EU fertilizer products is verified and certified to meet the requirements of FPR. Manufacturers of PBs should submit an EU-type examination to a single notified body of their choice, and the information and objects to be submitted include an application for type examination, technical documentation, product samples and supporting evidence of the technical design solution. The technical documentation should contain at least a general description of the product, a list of all constituent substances (their origins) and manufacturing processes, samples of labels or instructions, test reports, general specifications or lists of harmonized standards, etc. The Notified Body is responsible for checking the technical documentation and supporting evidence, preparing the assessment report and issuing the EU-Type Examination Certificate. Within 5 years after the product is placed on the market, the manufacturer shall retain a copy of the EU type test certificate and technical dossier to the national authority.
Module C is the inspection for internal production control. The manufacturer shall ensure that the manufacturing process of the product complies with the conditions approved by the type examination certificate. Each individual package of the product must affix the CE mark. The manufacturer shall also prepare an EU Declaration of Conformity, which shall be kept together with the technical documentation for a period of 5 years.
To verify that the efficacy claimed for products indeed falls within the definition of PBs, the European Commission has requested the European Committee for Standardization (CEN) to develop a European standard for PBs, which will serve as a basis for conformity assessment. The BS EN 17700 series of PBs – claims consists of 5 parts, including general principles, nutrient use efficiency, tolerance to abiotic stresses, determination of quality traits, and determination of availability of confined nutrients in the soil or rhizosphere. The general principles of this standard primarily outline the basic principles that should be followed when making claims about PBs products, including definition and scope, claim substantiation, transparency and communication, and regulatory considerations (EN 17700-2:2024, European Committee for Standardization). The other 4 sections provide scientific evidence on how to substantiate their claimed efficacy, positively list the applicable test indicators, and emphasize the importance of proper trial design, statistical analysis, and documentation and record keeping ensuring the correctness and traceability of all information. Products that have been registered as EU plant biostimulants include SURNAN®, Prime bio CE ®, AMALGEROL®, Biimore®, etc.
4) Post the CE mark and draft an EU Declaration of Conformity
After the PBs product has passed the conformity assessment and before the product is launched, the manufacturer should affix a label containing the CE mark on the product packaging and write an EU declaration of conformity. The main content of the EU Declaration of Conformity is detailed in FPR Annex V. It includes the EU fertilizer product (product number, batch number or type number), the name and address of the manufacturer and its authorized representative, the object of the declaration (product label image), the purpose of the declaration complies with FPR and other applicable EU harmonized legislation, notified bodies (name, number), certificate or approval number or other information. The manufacturer must retain the technical documentation and the EU Declaration of Conformity for at least 5 years after placing the product on the market.
Furthermore, according to the FPR, Chapter 5 regulates the control and safeguard mechanisms for EU fertilizer products entering the EU market. The purpose of this regulation is to ensure that products continuously comply with the requirements of the FPR throughout their lifecycle and to prevent non-compliant labeling, thereby maintaining the integrity of the regulation.
EUROPEAN BIOSTIMULANTS INDUSTRY COUNCIL'S ADVICE ON PBS COMPLIANCE
As global trade in agricultural products grows, the harmonization of PBs regulations is becoming increasingly important. To ensure the consistency of global standards and facilitate the flow of international markets, in addition to the European Union, other countries have also initiated regulatory regimes to plan PBs. Although FPR provides a comprehensive framework, there are still some challenges in ensuring PBs are compliant. EBIC actively communicates and coordinates with regulators on issues such as the identification of functional categories of fertilizers, restrictions on constituent substance categories, digitization of labels, and regulatory simplification and cost (European Biostimulants Industry Council, 2025).
1. Component materials of PBs:
1) REACH Regulation registration burden:
Although most of the main components of PBs are natural substances, registration in the EU is still subject to the REACH (Regulation (EC) 1907/2006) regulation. For example, the special specifications in Annex VIII, which include registration, notification, supply chain communication, and restrictions, are considered disproportionate and administratively burdensome. The EBIC calls for a reassessment of these requirements, while recommending the recognition of small-scale exemptions, streamlining chemical safety reporting, and minimizing duplication of regulations.
2) Expansion of permitted component materials for registration:
Many PBs based on microbial and animal by-products are currently excluded from the FPR. EBIC advocates for the inclusion of these components in the FPR framework in response to advances in new technologies and materials as well as market demand.
・Micro-organisms (CMC7):
The current FPR only allows four cultures, namely Azotobacter spp., Mycorrhizal fungi, Rhizobium spp. and Azospirillum spp., to be used for the registration of EU fertilizer products. Some scholars believe that beneficial micro-organisms such as Plant growth-promoting bacteria (PGPB) are relatively safe for mammals. According to Annex I of the FPR, the heavy metals and pathogens of this microorganism-plant biostimulant only need to be below the limit values specified in the FPR to meet the safety requirements, and there is no explicit requirement for toxicology data. However, there are still particular species or strains that may cause diseases in animals and humans and should be tested before they are applied (Barros-Rodríguez et al., 2020; Kumar et al., 2023). In the revised version of FPR on November 20, 2024, Article 42(4) of Chapter 6 provides clearer regulations for the addition of new microbial strains. The information that manufacturers must provide includes the natural occurrence, survival, and mobility of these microbial strains in the environment; scientific literature on the microbial production process, preservation, and use; documents regarding microbial residues, intermediates, toxins or microbial metabolites, and meeting the qualified safety presumption requirements set by the European Food Safety Authority. Suppose the scientific evidence for the new species or strains meet the requirements of paragraph 1(b), indicating that the micro-organism has agronomic efficiency and does not pose a risk to human, animal, or plant health, safety, or the environment. In that case, it qualifies for application as a component substance of fertilizer products.
- The dilemma of compliance for animal-derived products (CMC 10):
Several scientific studies on animal-derived products have shown effectiveness in promoting crop utilization of fertilizers, improving crop quality, and increasing yields, such as the significant ps and fish protein hydrolysates (He et al., 2024; Madende and Hayes 2022). However, it is problematic to limit the conversion process of protein hydrolysates within the range of processes approved for animal feed. Currently, animal-derived product-based PBs cannot be registered as EU fertilizer products, mainly due to the lack of a clear list of animal-derived products and the definition of "end point" status (excluding processed manure) in the FPR. EBIC recommends adopting a standards-based approach to ensure safety while providing greater flexibility for the use of protein hydrolysates in fertilizer products, ensuring that animal hydrolyzed proteins and products like chitosan can be applied to PBs.
2. Boundary cases of product functional categories:
Dual-use products are subject to regulatory uncertainty (e.g., products with both PBs and pesticide functions). Article 23 of the preamble of the FPR states that “Products with one or more functions, one of which is covered by the scope of Regulation (EC) No 1107/2009, are plant protection products falling within the scope of that Regulation. Those products should remain under the control developed for such products and provided for by that Regulation. Where such products also have the function of a fertilizing product, it would be misleading to provide for their CE marking under this Regulation, since the making available on the market of a plant protection product is contingent on a product authorization valid in the Member State concerned. Therefore, such products should be excluded from the scope of this Regulation”. This note restricts the registration of multifunctional products as PBs in the EU.
3. Digital labeling: EBIC recommends the introduction of digital labeling to make fertilizer product information more transparent and accessible.
4. Conformity assessment: Some EU member states may impose additional national requirements, leading to inconsistencies in the application of the FPR. EBIC recommends expanding the list of approved components and adopting a criteria-based approach to facilitate the inclusion of new and innovative products.
5. Reducing Unjustified Regulatory Costs:EBIC highlights the main reasons why the FPR imposes excessive costs on plant biostimulant manufacturers:
- Regulatory factors
- Extra REACH+ requirements: Beyond standard obligations, adding documentation and registration costs.
- Lack of harmonized standards: No unified testing methods; divergent national interpretations cause duplicate compliance.
- Heavy SME burden: Limited resources make obligations harder to meet.
- Raw material factors
- Microorganism restrictions: No clear approval pathway, hindering innovation.
- Recycled materials challenges: Lengthy procedures and high costs for by-products, limiting circular economy potential.
- Limited raw materials: Some excluded or require costly re-registration, raising costs and reducing flexibility.
- Registration & market factors
- Market entry delays: Complex, lengthy conformity assessments slow product launch.
CONCLUSION
Plant biostimulants (PBs) have been marketed in various countries for many years. According to global market projections, the PBs market value is expected to reach US$7.6 billion by 2029. In 2019, the EU Fertilizing Products Regulation (FPR) formally categorized PBs as fertilizing products and established a rigorous assessment framework. Although specific challenges remain regarding the registration thresholds for PBs, it is anticipated that the FPR will continue to be refined through ongoing coordination and regulatory amendments.
At present, the global definitions and regulatory frameworks for PBs are gradually converging, which facilitates international product circulation. The 2018 Farm Bill first referenced plant biostimulants (PBs) in U.S. congressional legislation, with USDA recommending a clear definition, science-based labeling standards, and a supportive regulatory framework. In 2023 and 2025, two versions of the Plant Biostimulant Act (S.802 and S.1907) were introduced to the Senate Agriculture, Nutrition, and Forestry Committee for review. S.802 aimed to amend the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), establish a unified definition for PBs, and exempt them from FIFRA regulation. S.1907 reintroduced FIFRA amendments, providing a unified and explicit definition of PBs: “a substance, microorganism, or mixture thereof, that, when applied to seeds, plants, the rhizosphere, soil, or other growth media, acts to support a plant’s natural processes independently of the nutrient content of that substance, microorganism, or mixture thereof, and that thereby improves nutrient availability, uptake, or use efficiency; tolerance to abiotic stress; and consequent growth, development, quality, or yield.” This definition is similar to the EU's, equivalent to the ISO definition. The bill requires the EPA, within 120 days of enactment, to amend and update the CFR (Code of Federal Regulations); it also directs the USDA to study the contributions of PBs to soil health and sustainability. This bill has not yet passed the committee. Industry stakeholders have proposed incorporating the content of S.1907 into a future Farm Bill.
In 2024, California enacted Senate Bill 1522 (SB 1522), becoming the first state in the U.S. to incorporate the content of AAPFCO’s Uniform Beneficial Substances Model Bill into state law. The bill amended the California Food and Agricultural Code by introducing a legal definition of PBs, updating labeling requirements, and revising the registration and licensing system. Currently, only materials containing humic acid, seaweed extract, or kelp extract are permitted to be registered as PBs. For other substances to be recognized as plant biostimulants, efficacy data must be reviewed and approved by California’s Fertilizing Materials Inspection Program (FMIP).
Similarly, India and South Africa have revised their fertilizer regulations to include PBs under regulatory oversight. Since 2021, Plant Biostimulants (PBs) have been formally brought within the scope of the Fertilizser (Inorganic, Organic or Mixed) (Control) Order, 1985 in India. The official notification defines Plant Biostimulants as follows: “Bio-stimulant means a substance or microorganism or a combination of both whose primary function, when applied to plants, seeds or the rhizosphere, is to stimulate physiological processes in plants and to enhance nutrient uptake, growth, yield, nutrient use efficiency, crop quality and tolerance to stress, regardless of its nutrient content, but does not include pesticides or plant growth regulators which are regulated under the Insecticides Act, 1968”. The categories of permissible ingredients comprise: botanical extracts, biochemicals, protein hydrolysates/amino acids, vitamins, cell-free microbial products, antioxidants, anti-transpirants, humic and fulvic acids, and live microorganisms (excluding biofertilizers and biopesticides). For the purpose of registration of a product as a Plant Biostimulant, the applicant is required to submit a chemical analysis report issued by a NABL-accredited or GLP-compliant laboratory, including details of active ingredient content, heavy metals, and impurities (including pesticide residues). Such data shall be verified by the referee/central laboratory as the basis for registration/eligibility determination.
Most Asian countries have not yet developed independent regulatory frameworks for PBs. In Japan, products whose primary components include seaweed extracts, humic acids, amino acids, or microorganisms, and which claim to improve soil conditions or enhance plant nutrient uptake, may be classified as special fertilizers or soil improvement materials. These categories are regulated under the Fertilizer Control Act and the Soil Fertility Enhancement Act. In Korea, products with PBs-like characteristics-such as organic, microbial, or soil-enzyme agents that promote soil health-are provisionally regulated as by-product fertilizers or soil activation agents under the Fertilizer Control Act. Such products are subject to requirements concerning minimum active ingredient content, maximum permissible levels of hazardous substances (e.g., heavy metals and other contaminants), shelf life, and labeling standards. In both Japan and Korea, products that claim to control pests, diseases, or weeds, or to regulate plant growth, are classified as pesticides and fall under the scope of pesticide legislation.
In Taiwan, the Agriculture and Food Agency, Ministry of Agriculture, is currently developing a regulatory framework for PBs. Ultimately, it is expected that only high-quality products that have successfully passed legal assessments will be registered as PBs, thereby improving crop yields and quality while ensuring the sustainable use of agricultural land.
REFERENCES
Barros-Rodríguez, A., P. Rangseekaew, K. Lasudee, W. Pathom-Aree and M. Manzanera. 2020. Regulatory risks associated with bacteria as biostimulants and biofertilizers in the frame of the European regulation (EU) 2019/1009. Sci. Total Environ. 740:140239. doi: 10.1016/j.scitotenv.2020.140239.
EN 17700-2:2024. Plant biostimulants – Claims – Nutrient use efficiency resulting from the use of a plant biostimulant. Brussels: European Committee for Standardization (CEN), 2024. Available at: https://www.en-standard.eu/bs-en-17700-2-2024-plant-biostimulants-claims... (accessed June 1, 2025).
EU. 2019. Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union, Document 32019R1009, OJ L 170, 25.6.2019, p. 1–114. (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02019R1009-2...)
European Biostimulants Industry Council (EBIC). Regulatory framework. Available at: https://biostimulants.eu/regulatory/ (accessed June 1, 2025).
Grow, D. G. 2022. FAQs Related to Regulation (EU) 2019/1009 on Fertilizing Products (the ‘Fertilizing Products Regulation’). European Comission - DG GROW.
He, F., Y. Tan, X. Zhou, T. Luo, Z. Yan, D. Xu and X. Wang. 2024. In-situ production of amino acid-rich monoammonium phosphate from chicken feathers provides superior efficacy compared to physical blending. Waste Management. 190: 273-284.
Kumari, M., P. Swarupa, K. K. Kesari and A. Kumar. 2023. Microbial inoculants as plant biostimulants: A review on risk status. Life 13, 12.
Madende, M. and M. Hayes. 2020. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules, 25, 1122.
van Schöll, L., W. H. Riechelman and R. Postma. 2023. Technical study on the elaboration of the technical documentation for the FPR. Inception report. Nutriënten Management Institute BV, Wageningen, Report 1935.N.22a, pp 65.
EU Fertilizing Products Regulation (FPR): Implications for the Global Development and Compliance of Plant Biostimulants
DOI: https://doi.org/10.56669/YRAU4781
ABSTRACT
The European Union Fertilizing Products Regulation (EU Regulation No 2019/2009, FPR) establishes a unified and stringent legal framework governing the approval, marketing, and use of fertilizing products, including plant biostimulants (PBs). Unlike its predecessor (EC Regulation No 2003/2003), which primarily focused on chemically synthesized inorganic fertilizers and lacked comprehensive oversight, the FPR expands regulatory coverage to include fertilizers derived from recycled organic materials while ensuring safety for human health and the environment. The PFR comprises eight chapters and fifty-three articles, addressing key areas such as the obligations of economic operators, product compliance, and notification by conformity assessment bodies. For the first time globally, PBs are formally defined and categorized under Product Function Category 6 (PFC 6), specifying four primary functions. Registration of PBs under the FPR requires compliance with product function definitions, composition categories, labelling requirements, and successful completion of conformity assessment procedures. Upon approval, products must bear the CE mark and submit an EU Declaration of Conformity, allowing free circulation within the EU market. However, several compliance challenges remain, including classification disputes, composition restrictions, digital labelling complexities, and administrative burdens. The European Biostimulants Industry Council (EBIC) continues to work closely with regulatory authorities to address these issues. The EU’s regulatory experience offers valuable insights for other regions, including the Asia-Pacific, as they seek to develop science-based, harmonized regulations that balance innovation, safety, and market accessibility for plant biostimulants.
Keywords: EU Fertilizing Products Regulation, plant biostimulants, definition, registration
OVERVIEW OF REGULATION (EU) No 2019/1009
The European Parliament and the Council of the European Union published Regulation (EU) No 2019/2009 (Fertilizing Products Regulation, FPR) on June 5, 2019, repealing the previous Regulation (EC) No 2003/2003 and amending the Regulation (EC) No 1107/2009; additionally, the revised current FPR was published on June 26, 2022, and is applicable from July 16, 2022 (EU, 2019). The main regulatory framework for regulating EU fertilizer products in conjunction with the FPR is listed in Table 1.
Table 1. The main regulatory framework of Regulation (EU) No 2019/1009.
EU Regulation
Main Regulatory Scope
Regulation (EC) 1907/2006
Regulates the registration, evaluation, authorization, and restriction of chemical substances to ensure safety for human health and the environment.
Regulation (EU) 1025/2012
Rules on the establishment of European standards.
Regulation (EC) No 1272/2008
EU regulations on the classification, labeling, and packaging of substances and mixtures.
Regulation (EC) No 1069/2009
Health rules on animal by-products
Regulation (EC) No 765/2008
Specifies requirements for CE certification of products, national certification bodies, and market surveillance.
Regulation (EU) No 2019/1020
Regulates market surveillance and compliance of products.
* In the EU, any chemical substance manufactured or imported in quantities exceeding 1 tonne per year must have its safety ensured by the manufacturer or importer, and it may only be placed on the EU market once registration has been completed. Annex II of the Fertilizing Products Regulation (EU 2019/1009) subjects CMCs 1, 6, 8, 11–15 to stricter requirements (“REACH+”) than those under REACH (EC 1907/2006) when substances are produced at <10 t/year for use in EU fertilizing products.
Regulation (EU) No 2019/2009 consists of 8 chapters and 53 articles and Appendix I-V, including general provisions, obligations of economic operators, conformity of EU fertilizing products, notification of conformity assessment bodies, Union market surveillance, delegated powers and committee procedure, amendments and transitional and final provisions. The regulations regarding the conditions and obligations of economic operators and conformity assessment bodies are fundamental, summarized as follows:
・ Before placing a product on the market, the manufacturer shall ensure that the product complies with the requirements of Annex I and II.
・ The manufacturer shall submit the technical documentation by the relevant conformity assessment procedures described in Article 15.
・ After successful conformity assessment, the manufacturer shall ensure that the product is accompanied by the labeling information required by Annex III.
・ Affix the CE mark on the product.
・ Preparation of an EU declaration of conformity.
・ Retain technical documentation and the EU Declaration of Conformity for 5 years after the product is placed on the market.
・ Provide copies of the EU statement of conformity to other economic operators.
・ EU member states shall designate a notified body as a conformity assessment body.
・ The notified body shall be established in accordance with the national laws of the member states, conducted by a national certification authority following the meanings and provisions of Regulation (EC) No 765/2008.
・ This notified body must be a third-party conformity assessment body with legal personality.
・ Requirements for personnel and subsidiaries responsible for conformity assessment tasks:
REQUIREMENTS FOR EU FERTILIZER PRODUCTS
Appendix I is a list of 7 functional categories (PFC1-7) of EU fertilizer products, including fertilizers (organic, organo-mineral fertilizers and inorganic fertilizers), liming material, soil improver, growing medium, inhibitors, plant biostimulants (PBs) and fertilizing product blend (i.e., products consisting of two or more EU fertilizer products from PFC 1 to PFC 6) (Table 2).
Table 2. Product functionality categories of EU fertilizers.
PFC 1
Fertiliser
A.
Organic fertiliser
I. Solid organic fertiliser
II. Liquid organic fertiliser
B.
Organo-mineral fertiliser
I. Solid organic fertiliser
II. Liquid organic fertiliser
C.
Inorganic fertiliser
I. Inorganic macronutrient fertiliser
II. Inorganic micronutrient fertiliser
PFC 2
Liming material
PFC 3
Soil improver
A.
Organic soil improver
B.
Inorganic soil improver
PFC 4
Growing medium
PFC 5
Inhibitor
A.
Nitrification inhibitor
B.
Denitrification inhibitor
C.
Urease inhibitor
PFC 6
Plant biostimulant
A.
Microbial plant biostimulant
B.
Non-microbial plant biostimulant
PFC 7
Fertilising product blend
An EU fertilizing product shall consist solely of component materials complying with the requirements for one or more of the CMCs listed in Annex II. There are 11 component material categories of the current FPR, including virgin material substances and mixtures (CMC1); plants, plant parts, or plant extracts (CMC2); compost (CMC3); fresh crop digestate (CMC4); digestate other than fresh crop digestate (CMC5); food industry by-products (CMC6); micro-organisms (CMC7); nutrient polymers (CMC8); polymers other than nutrient polymers (CMC9); Derived products within the meaning of Regulation (EC) No 1069/2009 (CMC 10) and by-products within the meaning of Directive 2008/98/EC (CMC11).
The November 20, 2024 revision of the FPR has been increased to 15 items, including precipitated phosphate salts and derivatives (CMC12), thermal oxidation materials and derivatives (CMC13), pyrolysis and gasification materials (CMC14), and recovered high-purity materials (CMC15) (Table 3). At the same time, animal-derived products (CMC10) have also been amended to allow the registration of Category 3 animal-derived substances as organic fertilizers and soil improvers if the product complies with Article 3(d) of the Delegated Regulation 2023/1605 (Supplementary Regulation (EU) No 1069/2009) to processed manure at the end of the manufacturing chain.
Table 3. Component material categories of EU fertilizers.
CMC 1: Virgin material substances and mixtures
CMC 2: Plants, plant parts or plant extracts
CMC 3: Compose
CMC 4: Fresh crop digestate
CMC 5: Digestate other than fresh crop digestate
CMC 6: Food industry by-products
CMC 7: micro-organisms
CMC 8: Nutrient polymers
CMC 9: Polymers other than nutrient polymers
CMC 10: Derived products within the meaning of Regulation (EC) No 1069/2009
CMC 11: By-products within the meaning of Directive 2008/98/EC
CMC 12: Precipitated phosphate salts and derivates
CMC 13: Thermal oxidation materials or derivates
CMC 14: Pyrolysis and gasification materials
CMC 15: Recovered high purity materials
3. Be labeled in accordance with the labeling requirements set out in Annex III
1) The product name and claimed functions as shown in Annex I.
2) the quantity of the EU fertilizing product, indicated by mass or volume.
3) Description of the expected use, including application rates, timing, frequency, and target plants.
4) Recommended storage conditions.
5) Products of CMC 9 polymers shall be in accordance with the instructions for use referred to in Part 2(d) of Annex II.
6) Any relevant information on measures recommended to manage risks to human, animal or plant health, to safety or the environment
7) A list of all ingredients exceeding 5%, arranged in descending order by dry weight; if the ingredient is a substance or mixture, it should be labeled according to Article 18 of Regulation (EC) No 1272/2008 on the classification, labeling, and packaging of substances and mixtures.
The labeling requirements for special products are based on the characteristics of different functional products, and separate specifications are formulated.
Tolerance rules
In FPR, the allowable values for the composition of different functional products are determined mainly due to possible deviations in manufacturing, sampling, and analysis of fertilizer products. The tolerances allowed in respect of the declared parameters indicated in this part include both negative and positive values.
4. Pass the conformity assessment inspection
In addition to meeting the previous three requirements, registered as an EU fertilizer product must pass the conformity assessment. The conformity assessment modules applicable to different functional products mainly depend on the functional category (PFC, Annex I) and its component materials category (CMC, Annex II). There are four types of modules A, A1, B+C and D1 for conformity assessment, of which the A1, B+C and D1 modules need to be certified by a notified body. For example, module A is suitable for internal production control of CMC1, 4, 6, 7, 11 and PFC 7, and should not be used for PFCs 1(C)(I)(a)(i-ii)(A), PFC5 and PFC6. Module A1 applies to PFC 1(C)(I)(a)(i-ii)(A) and PFC 1(C)(Iii)(A) for internal production control and supervised product testing. Module B is suitable for EU-type examination and module C is ideal for type conformity review for internal production control, so module B+C is suitable for one or more constituent products of CMC1, CMC2, CMC7, CMC9, CMC10, PFC5, PFC6 and PFC7. Module D1 is a quality assurance assessment for the production process and can be applied to any EU fertilizer products (except ammonia nitrate fertilizers) (Fig. 1) (Grow, 2022; van Schöll et al., 2023).
REGULATION OF EU PLANT BIOSTIMULANTS
1. Definition of PBs
Plant biostimulants are classified under product function category 6 (PFC 6) in FPR, with the functional requirements for PBs clarified in Appendix I: ‟an EU fertilizing product the function of which is to stimulate plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency, (b) tolerance to abiotic stress, (c) quality traits, or (d) availability of confined nutrients in the soil or rhizosphere. ”
2. Rationale for attributing PBs to fertilizers
According to point 22 of the preamble of FPR: ‟plant biostimulants are not as such inputs of nutrients, but stimulate plants’ natural nutrition processes. Where such products aim solely at improving the plants’ nutrient use efficiency, tolerance to abiotic stress, quality traits or increasing the availability of confined nutrients in the soil or rhizosphere, they are by nature more like fertilizing products than to most categories of plant protection products. They act in addition to fertilizers, intending to optimise the efficiency of those fertilizers and reduce the nutrient application rates. Such products should therefore be eligible for CE marking under this Regulation and shall not fall within the scope of Regulation (EC) No 1107/2009.” As a result, the European Parliament and the Council have classified PBs in the PFC 6 category under Regulation (EU) 2019/1009.
3. Requirements for registration as an EU PBs
1) Commonly used components materials of PBs
At present, the main components of commercially available PBs include amino acids, hydrolyzed proteins, seaweed extracts, humic acid, fulvic acid, micro-organisms, chitosan, etc., which correspond to the CMC of FPR and can be covered in the categories of CMC1, CMC2, CMC6, CMC7, CMC8, and CMC11. According to Part II Requirements related to CMCs, in which CMC1, CMC6, CMC8 and CMC11, whether used alone or in a mixture, should be registered in accordance with Regulation (EC) 1907/2006 (Registration, Evaluation and Authorization of Chemical Substances, REACH). The required documentation includes the technical documentation required by Article 10 of Annex VI, information requirements from Annex VII or VIII (depending on production or import volume), and the chemical safety report stipulated in Article 14 of the regulation. The purpose of this regulation is to protect human health and the environment from the hazards of chemical substances.
As animal-based hydrolyzed protein is an animal-derived product, it is currently regulated by Regulation (EC) No 1069/2009 and has not yet been included in the CMC10 of the current FPR. Therefore, PBs with animal hydrolyzed protein as the main ingredient cannot be registered as EU PBs products at present.
To ensure that EU fertilizer commodities do not adversely affect human and animal health and environmental safety, the levels of heavy metals in PBs and pathogen levels in microbial PBs and non-microbial PBs are limited in the FPR as shown in Table 4-6.
Table 4. Contaminants in a plant biostimulants must not exceed the limit.
metal
mg kg-1 dry matter
cadmium (Cd)
1.5
hexavalent chromium (Cr VI)
2
lead (Pb)
120
mercury (Hg)
1
nickel (Ni)
50
inorganic arsenic (As)
40
copper (Cu)
600
zinc (Zn)
500
Table 5. Pathogens in microbial plant biostimulants must not exceed the limits.
Micro-organisms/their toxins, metabolites
Sampling plans
Limit
n
c
Salmonella spp.
5
0
Absence in 25 g or 25 ml
Escherichia coli
5
0
Absence in 1 g or 1 ml
Listeria monocytogenes
5
0
Absence in 25 g or 25 ml
Vibrio spp.
5
0
Absence in 25 g or 25 ml
Shigella spp.
5
0
Absence in 25 g or 25 ml
Staphylococcus aureus
5
0
Absence in 25 g or 25 ml
Enterococcaceae
5
2
10 CFU/g
Anaerobic plate count unless the microbial plant biostimulant is an aerobic bacterium
5
2
105 CFU/g or ml
Yeast and mould count unless the microbial plant biostimulant is a fungus
5
2
1 000 CFU/g or ml
n = number of units comprising the sample,
c = number of sample units giving values over the defined limit.
Table 6. Pathogens in a non-microbial plant biostimulants must not exceed the limits.
Micro-organisms to be tested
Sampling plans
Limit
n
c
m
M
Salmonella spp.
5
0
0
Absence in 25 g or 25 ml
Escherichia coli or Enterococcaceae
5
5
0
1 000 in 1 g or 1 ml
n= number of samples to be tested,
c = number of samples where the number of bacteria expressed in CFU is between m and M,
m= threshold value for the number of bacteria expressed in CFU that is considered satisfactory,
M = maximum value of the number of bacteria expressed in CFU.
2) Labeling requirements
In addition to the general labeling requirements, the label of PBs must provide information including physical form, expiration date, method of application, claimed efficacy to the target plant, and any description of the efficacy of the product, including soil management measures, chemical fertilizers, incompatibility with pesticides, recommended nozzle size, aerosol pressure, and other anti-drift measures. Microbial PBs (PFC 6 (A)) should also be labelled for all added micro-organisms at levels expressed in active units per volume or weight, or in colony forming units per gram (cfu/g), and the following warning may be included on the label: “Microorganisms may have the potential to cause sensitization reactions”.
Tolerance values of PBs: Microbial PBs (PFC 6 (A)) and Non-Microbial PBs (PFC 6 (B)) have allowable tolerances of ±15 % and ±5 %, respectively. If the fertilizer product blend (PFC 7) contains one or more PBs, the concentration of each PB is expressed in g/kg or g/l.
3) Conformity assessment of PBs is applicable to the B+C module
Follows the FPR specifications, and the qualified assessment module B+C is suitable for the testing of PBs. The conformity assessment includes testing, inspection and certification, and must pass a conformity assessment to ensure that the product meets safety, effectiveness and environmental standards, as shown in Fig. 2 (van Schöll et al., 2023).
Module B is part of the EU-type examination, in which the technical design of EU fertilizer products is reviewed by a Notified Body and the technical design of EU fertilizer products is verified and certified to meet the requirements of FPR. Manufacturers of PBs should submit an EU-type examination to a single notified body of their choice, and the information and objects to be submitted include an application for type examination, technical documentation, product samples and supporting evidence of the technical design solution. The technical documentation should contain at least a general description of the product, a list of all constituent substances (their origins) and manufacturing processes, samples of labels or instructions, test reports, general specifications or lists of harmonized standards, etc. The Notified Body is responsible for checking the technical documentation and supporting evidence, preparing the assessment report and issuing the EU-Type Examination Certificate. Within 5 years after the product is placed on the market, the manufacturer shall retain a copy of the EU type test certificate and technical dossier to the national authority.
Module C is the inspection for internal production control. The manufacturer shall ensure that the manufacturing process of the product complies with the conditions approved by the type examination certificate. Each individual package of the product must affix the CE mark. The manufacturer shall also prepare an EU Declaration of Conformity, which shall be kept together with the technical documentation for a period of 5 years.
To verify that the efficacy claimed for products indeed falls within the definition of PBs, the European Commission has requested the European Committee for Standardization (CEN) to develop a European standard for PBs, which will serve as a basis for conformity assessment. The BS EN 17700 series of PBs – claims consists of 5 parts, including general principles, nutrient use efficiency, tolerance to abiotic stresses, determination of quality traits, and determination of availability of confined nutrients in the soil or rhizosphere. The general principles of this standard primarily outline the basic principles that should be followed when making claims about PBs products, including definition and scope, claim substantiation, transparency and communication, and regulatory considerations (EN 17700-2:2024, European Committee for Standardization). The other 4 sections provide scientific evidence on how to substantiate their claimed efficacy, positively list the applicable test indicators, and emphasize the importance of proper trial design, statistical analysis, and documentation and record keeping ensuring the correctness and traceability of all information. Products that have been registered as EU plant biostimulants include SURNAN®, Prime bio CE ®, AMALGEROL®, Biimore®, etc.
4) Post the CE mark and draft an EU Declaration of Conformity
After the PBs product has passed the conformity assessment and before the product is launched, the manufacturer should affix a label containing the CE mark on the product packaging and write an EU declaration of conformity. The main content of the EU Declaration of Conformity is detailed in FPR Annex V. It includes the EU fertilizer product (product number, batch number or type number), the name and address of the manufacturer and its authorized representative, the object of the declaration (product label image), the purpose of the declaration complies with FPR and other applicable EU harmonized legislation, notified bodies (name, number), certificate or approval number or other information. The manufacturer must retain the technical documentation and the EU Declaration of Conformity for at least 5 years after placing the product on the market.
Furthermore, according to the FPR, Chapter 5 regulates the control and safeguard mechanisms for EU fertilizer products entering the EU market. The purpose of this regulation is to ensure that products continuously comply with the requirements of the FPR throughout their lifecycle and to prevent non-compliant labeling, thereby maintaining the integrity of the regulation.
EUROPEAN BIOSTIMULANTS INDUSTRY COUNCIL'S ADVICE ON PBS COMPLIANCE
As global trade in agricultural products grows, the harmonization of PBs regulations is becoming increasingly important. To ensure the consistency of global standards and facilitate the flow of international markets, in addition to the European Union, other countries have also initiated regulatory regimes to plan PBs. Although FPR provides a comprehensive framework, there are still some challenges in ensuring PBs are compliant. EBIC actively communicates and coordinates with regulators on issues such as the identification of functional categories of fertilizers, restrictions on constituent substance categories, digitization of labels, and regulatory simplification and cost (European Biostimulants Industry Council, 2025).
1. Component materials of PBs:
1) REACH Regulation registration burden:
Although most of the main components of PBs are natural substances, registration in the EU is still subject to the REACH (Regulation (EC) 1907/2006) regulation. For example, the special specifications in Annex VIII, which include registration, notification, supply chain communication, and restrictions, are considered disproportionate and administratively burdensome. The EBIC calls for a reassessment of these requirements, while recommending the recognition of small-scale exemptions, streamlining chemical safety reporting, and minimizing duplication of regulations.
2) Expansion of permitted component materials for registration:
Many PBs based on microbial and animal by-products are currently excluded from the FPR. EBIC advocates for the inclusion of these components in the FPR framework in response to advances in new technologies and materials as well as market demand.
・Micro-organisms (CMC7):
The current FPR only allows four cultures, namely Azotobacter spp., Mycorrhizal fungi, Rhizobium spp. and Azospirillum spp., to be used for the registration of EU fertilizer products. Some scholars believe that beneficial micro-organisms such as Plant growth-promoting bacteria (PGPB) are relatively safe for mammals. According to Annex I of the FPR, the heavy metals and pathogens of this microorganism-plant biostimulant only need to be below the limit values specified in the FPR to meet the safety requirements, and there is no explicit requirement for toxicology data. However, there are still particular species or strains that may cause diseases in animals and humans and should be tested before they are applied (Barros-Rodríguez et al., 2020; Kumar et al., 2023). In the revised version of FPR on November 20, 2024, Article 42(4) of Chapter 6 provides clearer regulations for the addition of new microbial strains. The information that manufacturers must provide includes the natural occurrence, survival, and mobility of these microbial strains in the environment; scientific literature on the microbial production process, preservation, and use; documents regarding microbial residues, intermediates, toxins or microbial metabolites, and meeting the qualified safety presumption requirements set by the European Food Safety Authority. Suppose the scientific evidence for the new species or strains meet the requirements of paragraph 1(b), indicating that the micro-organism has agronomic efficiency and does not pose a risk to human, animal, or plant health, safety, or the environment. In that case, it qualifies for application as a component substance of fertilizer products.
Several scientific studies on animal-derived products have shown effectiveness in promoting crop utilization of fertilizers, improving crop quality, and increasing yields, such as the significant ps and fish protein hydrolysates (He et al., 2024; Madende and Hayes 2022). However, it is problematic to limit the conversion process of protein hydrolysates within the range of processes approved for animal feed. Currently, animal-derived product-based PBs cannot be registered as EU fertilizer products, mainly due to the lack of a clear list of animal-derived products and the definition of "end point" status (excluding processed manure) in the FPR. EBIC recommends adopting a standards-based approach to ensure safety while providing greater flexibility for the use of protein hydrolysates in fertilizer products, ensuring that animal hydrolyzed proteins and products like chitosan can be applied to PBs.
2. Boundary cases of product functional categories:
Dual-use products are subject to regulatory uncertainty (e.g., products with both PBs and pesticide functions). Article 23 of the preamble of the FPR states that “Products with one or more functions, one of which is covered by the scope of Regulation (EC) No 1107/2009, are plant protection products falling within the scope of that Regulation. Those products should remain under the control developed for such products and provided for by that Regulation. Where such products also have the function of a fertilizing product, it would be misleading to provide for their CE marking under this Regulation, since the making available on the market of a plant protection product is contingent on a product authorization valid in the Member State concerned. Therefore, such products should be excluded from the scope of this Regulation”. This note restricts the registration of multifunctional products as PBs in the EU.
3. Digital labeling: EBIC recommends the introduction of digital labeling to make fertilizer product information more transparent and accessible.
4. Conformity assessment: Some EU member states may impose additional national requirements, leading to inconsistencies in the application of the FPR. EBIC recommends expanding the list of approved components and adopting a criteria-based approach to facilitate the inclusion of new and innovative products.
5. Reducing Unjustified Regulatory Costs:EBIC highlights the main reasons why the FPR imposes excessive costs on plant biostimulant manufacturers:
CONCLUSION
Plant biostimulants (PBs) have been marketed in various countries for many years. According to global market projections, the PBs market value is expected to reach US$7.6 billion by 2029. In 2019, the EU Fertilizing Products Regulation (FPR) formally categorized PBs as fertilizing products and established a rigorous assessment framework. Although specific challenges remain regarding the registration thresholds for PBs, it is anticipated that the FPR will continue to be refined through ongoing coordination and regulatory amendments.
At present, the global definitions and regulatory frameworks for PBs are gradually converging, which facilitates international product circulation. The 2018 Farm Bill first referenced plant biostimulants (PBs) in U.S. congressional legislation, with USDA recommending a clear definition, science-based labeling standards, and a supportive regulatory framework. In 2023 and 2025, two versions of the Plant Biostimulant Act (S.802 and S.1907) were introduced to the Senate Agriculture, Nutrition, and Forestry Committee for review. S.802 aimed to amend the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), establish a unified definition for PBs, and exempt them from FIFRA regulation. S.1907 reintroduced FIFRA amendments, providing a unified and explicit definition of PBs: “a substance, microorganism, or mixture thereof, that, when applied to seeds, plants, the rhizosphere, soil, or other growth media, acts to support a plant’s natural processes independently of the nutrient content of that substance, microorganism, or mixture thereof, and that thereby improves nutrient availability, uptake, or use efficiency; tolerance to abiotic stress; and consequent growth, development, quality, or yield.” This definition is similar to the EU's, equivalent to the ISO definition. The bill requires the EPA, within 120 days of enactment, to amend and update the CFR (Code of Federal Regulations); it also directs the USDA to study the contributions of PBs to soil health and sustainability. This bill has not yet passed the committee. Industry stakeholders have proposed incorporating the content of S.1907 into a future Farm Bill.
In 2024, California enacted Senate Bill 1522 (SB 1522), becoming the first state in the U.S. to incorporate the content of AAPFCO’s Uniform Beneficial Substances Model Bill into state law. The bill amended the California Food and Agricultural Code by introducing a legal definition of PBs, updating labeling requirements, and revising the registration and licensing system. Currently, only materials containing humic acid, seaweed extract, or kelp extract are permitted to be registered as PBs. For other substances to be recognized as plant biostimulants, efficacy data must be reviewed and approved by California’s Fertilizing Materials Inspection Program (FMIP).
Similarly, India and South Africa have revised their fertilizer regulations to include PBs under regulatory oversight. Since 2021, Plant Biostimulants (PBs) have been formally brought within the scope of the Fertilizser (Inorganic, Organic or Mixed) (Control) Order, 1985 in India. The official notification defines Plant Biostimulants as follows: “Bio-stimulant means a substance or microorganism or a combination of both whose primary function, when applied to plants, seeds or the rhizosphere, is to stimulate physiological processes in plants and to enhance nutrient uptake, growth, yield, nutrient use efficiency, crop quality and tolerance to stress, regardless of its nutrient content, but does not include pesticides or plant growth regulators which are regulated under the Insecticides Act, 1968”. The categories of permissible ingredients comprise: botanical extracts, biochemicals, protein hydrolysates/amino acids, vitamins, cell-free microbial products, antioxidants, anti-transpirants, humic and fulvic acids, and live microorganisms (excluding biofertilizers and biopesticides). For the purpose of registration of a product as a Plant Biostimulant, the applicant is required to submit a chemical analysis report issued by a NABL-accredited or GLP-compliant laboratory, including details of active ingredient content, heavy metals, and impurities (including pesticide residues). Such data shall be verified by the referee/central laboratory as the basis for registration/eligibility determination.
Most Asian countries have not yet developed independent regulatory frameworks for PBs. In Japan, products whose primary components include seaweed extracts, humic acids, amino acids, or microorganisms, and which claim to improve soil conditions or enhance plant nutrient uptake, may be classified as special fertilizers or soil improvement materials. These categories are regulated under the Fertilizer Control Act and the Soil Fertility Enhancement Act. In Korea, products with PBs-like characteristics-such as organic, microbial, or soil-enzyme agents that promote soil health-are provisionally regulated as by-product fertilizers or soil activation agents under the Fertilizer Control Act. Such products are subject to requirements concerning minimum active ingredient content, maximum permissible levels of hazardous substances (e.g., heavy metals and other contaminants), shelf life, and labeling standards. In both Japan and Korea, products that claim to control pests, diseases, or weeds, or to regulate plant growth, are classified as pesticides and fall under the scope of pesticide legislation.
In Taiwan, the Agriculture and Food Agency, Ministry of Agriculture, is currently developing a regulatory framework for PBs. Ultimately, it is expected that only high-quality products that have successfully passed legal assessments will be registered as PBs, thereby improving crop yields and quality while ensuring the sustainable use of agricultural land.
REFERENCES
Barros-Rodríguez, A., P. Rangseekaew, K. Lasudee, W. Pathom-Aree and M. Manzanera. 2020. Regulatory risks associated with bacteria as biostimulants and biofertilizers in the frame of the European regulation (EU) 2019/1009. Sci. Total Environ. 740:140239. doi: 10.1016/j.scitotenv.2020.140239.
EN 17700-2:2024. Plant biostimulants – Claims – Nutrient use efficiency resulting from the use of a plant biostimulant. Brussels: European Committee for Standardization (CEN), 2024. Available at: https://www.en-standard.eu/bs-en-17700-2-2024-plant-biostimulants-claims... (accessed June 1, 2025).
EU. 2019. Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union, Document 32019R1009, OJ L 170, 25.6.2019, p. 1–114. (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02019R1009-2...)
European Biostimulants Industry Council (EBIC). Regulatory framework. Available at: https://biostimulants.eu/regulatory/ (accessed June 1, 2025).
Grow, D. G. 2022. FAQs Related to Regulation (EU) 2019/1009 on Fertilizing Products (the ‘Fertilizing Products Regulation’). European Comission - DG GROW.
He, F., Y. Tan, X. Zhou, T. Luo, Z. Yan, D. Xu and X. Wang. 2024. In-situ production of amino acid-rich monoammonium phosphate from chicken feathers provides superior efficacy compared to physical blending. Waste Management. 190: 273-284.
Kumari, M., P. Swarupa, K. K. Kesari and A. Kumar. 2023. Microbial inoculants as plant biostimulants: A review on risk status. Life 13, 12.
Madende, M. and M. Hayes. 2020. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules, 25, 1122.
van Schöll, L., W. H. Riechelman and R. Postma. 2023. Technical study on the elaboration of the technical documentation for the FPR. Inception report. Nutriënten Management Institute BV, Wageningen, Report 1935.N.22a, pp 65.