DOI: https://doi.org/10.56669/ZFNY1949
ABSTRACT
Acid tolerant bacteria were isolated and screened for growth promoting activities in vitro and in the growth room. Acid tolerant PGPB 84, a nitrogen fixer, and PGPB 89, a phosphate solubilizer, were selected for further evaluation. In field trials in acidic soils, significant response to PGPB inoculation was obtained with full fertilizer rate. In acidic Luisiana clay, PGPB 89 increased average total fruit weight of hot pepper cv Aruy uy by 105% and average total fruit number by 96% across fertilizer treatment. The application of the acid tolerant PGPB 89 inoculant in Luisiana clay had no significant effect on soil pH, organic matter, exchangeable K and soil microbial biomass but exchangeable P was significantly reduced.
Keywords: PGPB, acid tolerant, eggplant, hot pepper
INTRODUCTION
Environmental concerns attributed to climate change, the use of chemical farm inputs and the rising cost of fossil fuel-derived fertilizers have increased interest in soil microorganisms as important biological resources for improving sustainability of crop production. With the advent of biotechnology, researchers are trying to find ways to use soil microorganisms to improve plant growth and to understand the mechanisms involved in plant-microbe interaction.
Climate change has triggered extreme weather conditions hence the need for climate change resilient microbial-based technology. There is a need to develop microbial inoculants tolerant to soil stresses such as acidity, salinity and drought to improve soil productivity with minimum chemical inputs.
In the Philippines, it is estimated that about 9.6 M ha of land have pH levels below 5.5 and low fertility and limiting crop yields. Soil acidity limits crop yields brought about by aluminum toxicity, phosphorus and calcium deficiency (Marschner, 1991).
Microbial inoculation of crops grown in acidic soils has so far improved crop productivity (Dutta et al., 2024; O’Callaghan et al., 2022). Early efforts on microbial inoculation of crops grown in acidic soils involved legume inoculation with acid tolerant rhizobia resulting in better survival and nodule competency over the acid intolerant strain (Graham et al., 1982). Inoculation of shrubby legumes grown in acid soils with acid tolerant rhizobia has improved plant growth, so the revegetation plan for disturbed areas in acidic soils of western Spain involving inoculating legume with acid tolerant rhizobia (Rodriguez-Echeverria and Perez-Fernandez, 2005). Local studies have also shown the benefit of utilizing acid tolerant rhizobia for improving crop productivity in acid soil under field and greenhouse conditions (Paterno et al.,1996; Delfin et al., 2003).
Low phosphorus availability is one of the common problems in acid soils. The strategies involved in increasing productivity under low P condition were increased use of P fertilizer and utilization of microbial-based technologies. Among the microbial-based technologies are inoculation with mycorrhiza and phosphate solubilizing bacteria. Mycorrhiza, specifically vesicular arbuscular mycorrhiza (VAM) enhanced the availability of P through enhanced uptake and translocation of phosphate ions from soil solution (Harley and Smith, 1983). On the other hand, phosphate solubilizing bacteria (PSB) solubilize insoluble P through organic acid production (Taha et al., 1969). Inoculation of tomato with VAM and PSB under low P soil increases plant growth, microbial biomass and alkaline phosphatase activity in the rhizosphere (Kim et al., 1998).
METHODOLOGY
Screening of acid tolerant isolates for promoting plant growth was conducted in the growth chambers. Solid based formulations of the selected isolates were prepared for screening. The isolates were initially grown in the acidified media used for their isolations. The cultures were then grown in the production medium for 48 hours. Sterilized solid based carrier consisting of a mixture of soil and charcoal were then inoculated with the cultures.
Twelve selected acid tolerant PGPB isolates which produced IAA and showed ACC deaminase activity were screened for plant growth promotion under gnotobiotic condition using eggplant (Solanum melongena L.) as test crop. The isolates consisted of seven nitrogen fixers (PGPB 56, 57, 60, 81, 82, 84, 85) and five phosphate solubilizers (PGPB 46, 49, 86, 87, 89). Sterile Magenta jars were prepared with acidic Luisiana clay as the growing medium. Simple Nutrient Addition Program (SNAPTM) solution was used as source of plant nutrients. The treatments also included an existing acid tolerant isolate (TCeRe9), and an uninoculated control, and were replicated four times.
A 1:10 sterile water suspension of the solid based preparations of the test isolates was used to inoculate seeds at the time of sowing and transplanting. Eggplant seeds were surface sterilized, sown in sterile vermiculite in aluminum trays and drenched with the inoculant suspension. When transplanting, the seedling roots were dipped in the inoculant suspension. Dry matter yield was measured four weeks after transplantation.
Field evaluation of selected acid tolerant PGPB: Field trials were established to evaluate the performance of selected acid tolerant PGPB strains 84 and 89 under field condition at the Cavinti Municipal Nursery, Cavinti, Laguna. Commercial eggplant variety ‘Violeta’ and hot pepper variety ‘Aruy-uy’ were used as test plants. Seeds of both crops were inoculated at sowing and reinoculated at time of pricking and transplanting by dipping the roots in 100g inoculant: 2Lwater suspension.
Soil samples from the area were collected and submitted for analysis. The results of the soil analyses are shown in Table 1 and were used as a basis for the rate of fertilizer applied in the trial. The experiment was a two-factorial arranged in RCBD with 3 replications. The two factors included in the trial were fertilizer rates: full recommended fertilizer rate and half recommended fertilizer rate; and inoculation: without inoculation, and inoculation with PGPB 84 and PGPB 89. The full rate used for hot pepper was 140-70-140 kg NPK per ha while the half rate was 70-35-70 kg NPK per ha. A small dose of fertilizer (0.5 g per plant for full rate and 0.25 g for half rate) was mixed with the soil medium placed in seedling trays for pricking of seedlings. When transplanting, plots with full fertilizer treatment were applied with complete fertilizer (14-14-14) equivalent to 70-70-70 NPK per ha while the plots with half fertilizer rate treatment were applied with complete fertilizer equivalent to 35-35-35 NPK per ha. The remaining fertilizer requirements are divided into 3WAT and 6WAT.
Similar treatments were initially applied to eggplant. However, an additional fertilizer was added to the trial at 3 months after transplantation to boost new leaf growth while premature leaf senescence was observed. Ten grams of complete fertilizer (14-14-14) was added to eggplants in the full fertilizer treatment while 5 grams in the half fertilizer treatment. The 10 g complete fertilizer added was equivalent to an additional 56 kg NPK for the full fertilizer treatment while the additional 5 g was equivalent to 28 kg NPK for the half fertilizer treatment. So far, only a few fruits were harvested from the area.
RESULTS AND DISCUSSION
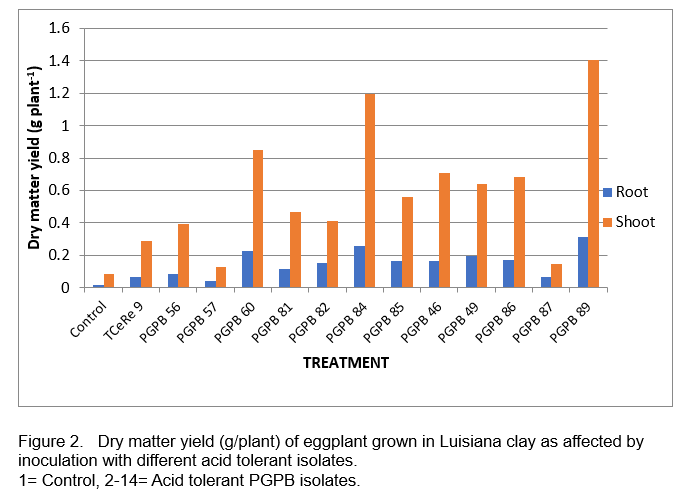
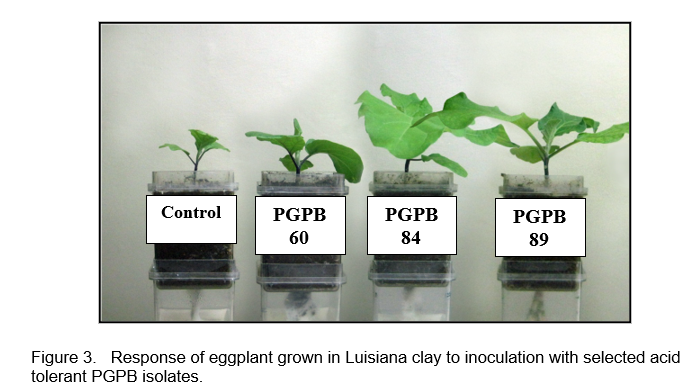
Screening of acid tolerant isolates for plant growth promotion was conducted in the growth chambers. Seven (7) nitrogen fixers (PGPB 56, 57, 60, 81, 82, 84, 85) and five (5) phosphate solubilizers (PGPB 46, 49, 86, 87, 89) which produced IAA and showed ACC deaminase activity were screened for plant growth promotion under gnotobiotic condition using eggplant (Solanum melongena L.) as test crop (Figure 1). Figure 2 shows the effect of inoculation with the different isolates on the dry matter yield of eggplant at four weeks after transplantation. PGPB 84 and 89 gave the highest dry matter yields (Figure 3). Isolates PGPB 84 and PGPB 89 increased shoot dry matter yield by 14- and 16-fold, respectively, over the control. These isolates were further evaluated in the field.

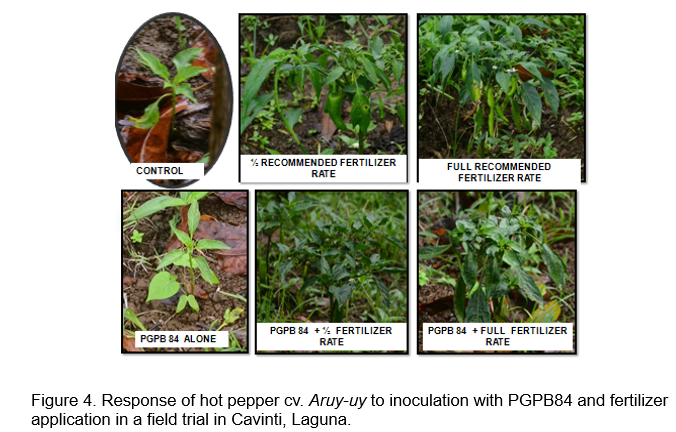
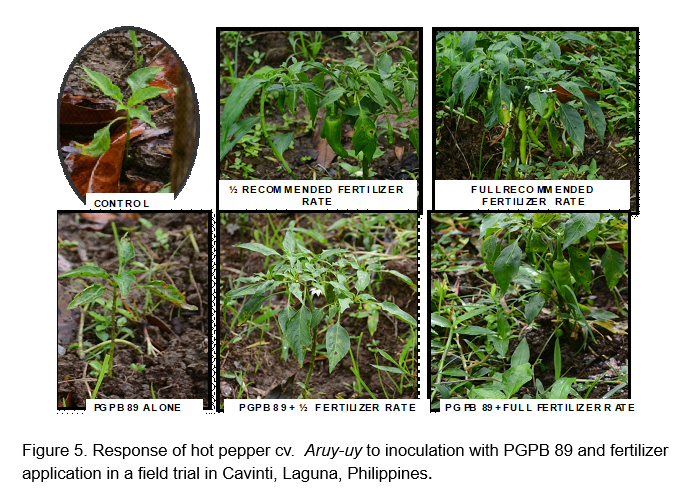
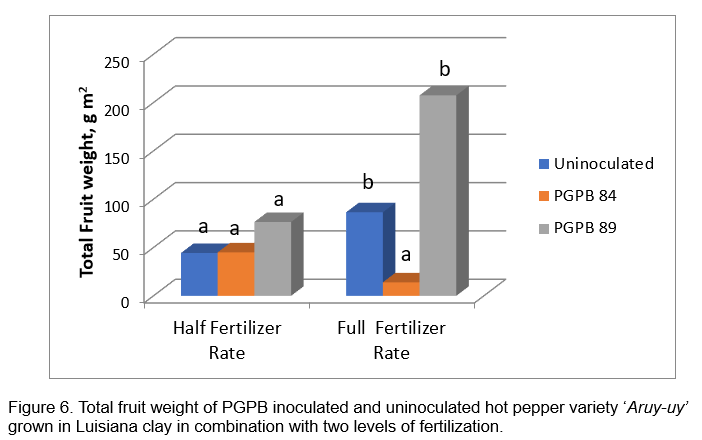
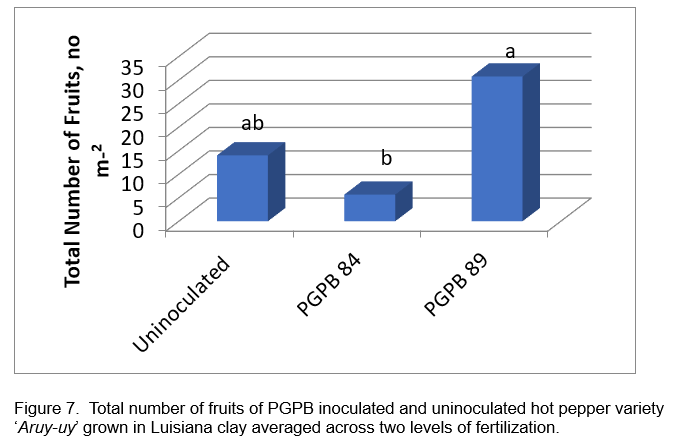
Field evaluation of selected acid tolerant PGPB 84 and 89: Figures 4 and 5 show the response of hot pepper cv. Aruy-uy to inoculation with acid tolerant PGPB. Fruit yield of hot pepper was obtained from 4 plants in the inner rows, with an area measurement of 1 square meter. A total of 3 primings were obtained from the field trial due to the early onset of leaf spot diseases which resulted in early leaf senescence. Statistical analysis of yield data showed significant fertilizer rate-inoculation interaction. Significant response to PGPB 89 inoculation was obtained with full fertilizer rate application with the highest harvest of 207.50 g m-2 (Figure 6). Inoculation with PGPB 89 increased average total fruit weight by 105% and average total fruit number by 96% across fertilizer treatment. Interestingly, PGPB 84 inoculated plants performed poorly than uninoculated hot pepper in the fully fertilized plots. PGPB 89 inoculated plants produced the highest number of fruits averaged across fertilizer rates (Figure 7). Fertilizer rate did not have significant effect on the number of fruits formed.
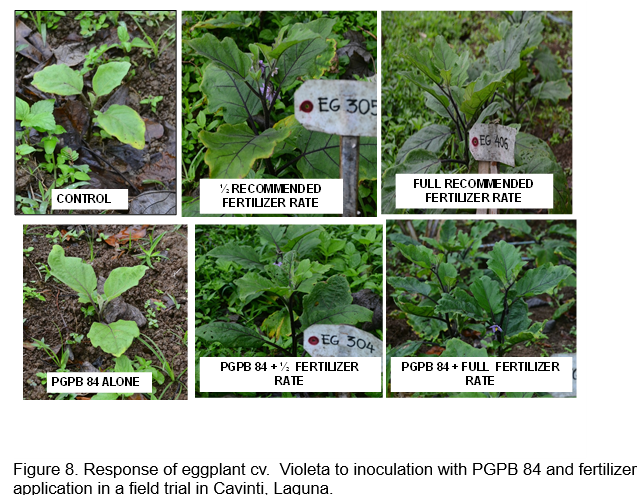
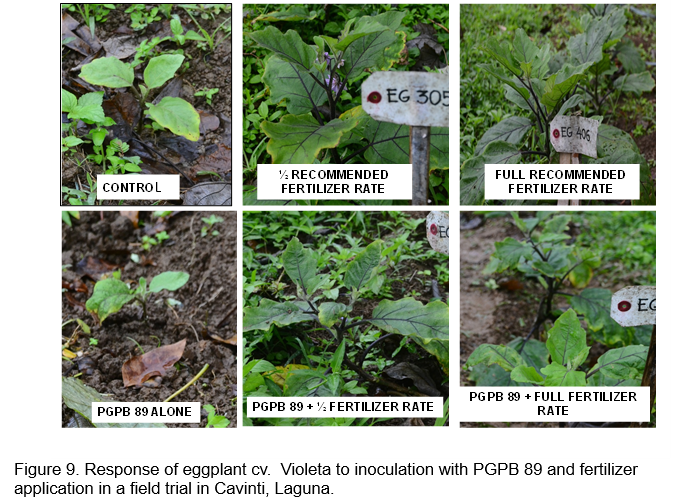
Figures 8 and 9 show the response of eggplant cv. Violeta to inoculation with acid tolerant PGPB. Similar treatments were initially applied for eggplant. However, additional fertilizer was added to the trial at 3 months after transplanting to boost new leaf growth since premature leaf senescence was observed. Ten grams of complete fertilizer (14-14-14) were added to eggplant in the full fertilizer treatment while 5 grams was added to the half fertilizer treatment. The 10 g complete fertilizer added was equivalent to an additional 56 kg NPK for the full fertilizer treatment while the additional 5 g was equivalent to 28 kg NPK for the half fertilizer treatment. However, only a few fruits were harvested from the area.
A significant response to PGPB 89 inoculation by hot pepper was obtained in combination with the application of full fertilizer. PGPB 89 could produce both ACC deaminase and IAA. Growth promotion by PGPB has been attributed to phytohormone production such as IAA. The IAA enhances the rapid establishment of roots in young seedlings which increases their survival through increased nutrient and water uptake (Patten and Glick, 2002).
The acid-tolerant PGPBs isolates were deposited in the Philippine National Collection of Microorganisms (PNCM). Future studies include packaging of the inoculant formulation for testing in other acidic soil in the country and eventual registration to the Fertilizer and Pesticide Authority (FPA).
CONCLUSION
Acid tolerant bacteria were isolated, characterized and evaluated for growth promoting activities. Results showed that supplementing the recommended fertilizer rate with stress tolerant inoculants significantly increased yield of hot pepper and eggplant grown in acidic soils. Further greenhouse and field trials should provide more information on PGPB-plant variety interaction. Moreover, the stress tolerant PGPB inoculants should be further evaluated as components of nutrient management strategies to improve the productivity of degraded soils. The effect of the application of acid tolerant PGPB inoculants on the chemical and biological properties of Luisiana clay was assessed. Soil pH, organic matter content, exchangeable potassium and soil microbial biomass were not significantly affected by inoculation. In contrast inoculation significantly reduced exchangeable phosphorus. This may be related to an increase in plant uptake of P because of improved plant growth and increased microbial activities. The interaction between the introduced microorganism as inoculants and the indigenous soil microbial population is likely to be complex. Hence, a better understanding of the mechanisms of this interaction is necessary to predict short- and long-term effects (Bϋnemann, et al., 2006). The data obtained on the effect of PGPB inoculation on the environment are too limited to be able to draw a conclusion. A more extensive study on the effect of microbial inoculants on microbial diversity using molecular techniques should be the focus of future research.
ACKNOWLEDGEMENT
We are grateful for the funding support of the Department of Science and Technology- Philippine Council for Industry, Energy, and Emerging Technology Research and Development (DOST-PCIEERD) and the generosity of our farmer collaborators.
REFERENCES
Delfin, E.F., E.S. Paterno, A.M. Ocampo and F.C. Rodriguez.. 2003. Growth, yield and nutrient uptake of mungbean (vigna radiata l.) grown in Antipolo clay amended with lime, nitrogen and phosphorus fertilizers and microbial inoculants. The Phil. Agricultural Scientist 86: 75-83.
Dutta, S.K., Sarma, H.H., Saud, R.K., Konwar, M.J., Gogoi, B., Pathak, K., Bora, S.K., Mahanta, S., 2024. Assessment of organic and natural farming practices on quality parameters of joha rice and soil microbial biomass carbon (SMBC) in soil under cultivation. Plant Archives 24 (2), 2164–2168.
Harley JL, Smith SE. 1983 Mycorrhizal symbiosis. Academic Press, London, pp 317–356.
Kim, K.Y., D. Jordan and G.A. McDonald. 1998. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol Fertil Soils 26:79–87.
Marschner, H. 1991 Mechanisms of adaptation of plants to acid soils. Plant Soil 134, 1–20.
O’Callaghan, M., Ballard, R. A. & Wright, D. 2022. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use and Management, 38, 1340–1369. https://doi.org/10.1111/sum.12811
Paterno, E.S. M.L.Q. Sison, F.G.Torres, E.S. Garcia, S.M. Pantua and M.E. Orlina. 1996. Development of Microbial Inoculants for Stressed Environment. Terminal Report. BIOTECH,UPLB-PCASTRD Project.
Patten C. and Glick B.R., 1996. Bacterial biosynthesis of indole-3-acetic acid. Canadian Journal of Microbiology 42: 207-220.
Preliminary Studies on the Acid Tolerance of Some Plant Growth Promoting Bacteria for Vegetable Production
DOI: https://doi.org/10.56669/ZFNY1949
ABSTRACT
Acid tolerant bacteria were isolated and screened for growth promoting activities in vitro and in the growth room. Acid tolerant PGPB 84, a nitrogen fixer, and PGPB 89, a phosphate solubilizer, were selected for further evaluation. In field trials in acidic soils, significant response to PGPB inoculation was obtained with full fertilizer rate. In acidic Luisiana clay, PGPB 89 increased average total fruit weight of hot pepper cv Aruy uy by 105% and average total fruit number by 96% across fertilizer treatment. The application of the acid tolerant PGPB 89 inoculant in Luisiana clay had no significant effect on soil pH, organic matter, exchangeable K and soil microbial biomass but exchangeable P was significantly reduced.
Keywords: PGPB, acid tolerant, eggplant, hot pepper
INTRODUCTION
Environmental concerns attributed to climate change, the use of chemical farm inputs and the rising cost of fossil fuel-derived fertilizers have increased interest in soil microorganisms as important biological resources for improving sustainability of crop production. With the advent of biotechnology, researchers are trying to find ways to use soil microorganisms to improve plant growth and to understand the mechanisms involved in plant-microbe interaction.
Climate change has triggered extreme weather conditions hence the need for climate change resilient microbial-based technology. There is a need to develop microbial inoculants tolerant to soil stresses such as acidity, salinity and drought to improve soil productivity with minimum chemical inputs.
In the Philippines, it is estimated that about 9.6 M ha of land have pH levels below 5.5 and low fertility and limiting crop yields. Soil acidity limits crop yields brought about by aluminum toxicity, phosphorus and calcium deficiency (Marschner, 1991).
Microbial inoculation of crops grown in acidic soils has so far improved crop productivity (Dutta et al., 2024; O’Callaghan et al., 2022). Early efforts on microbial inoculation of crops grown in acidic soils involved legume inoculation with acid tolerant rhizobia resulting in better survival and nodule competency over the acid intolerant strain (Graham et al., 1982). Inoculation of shrubby legumes grown in acid soils with acid tolerant rhizobia has improved plant growth, so the revegetation plan for disturbed areas in acidic soils of western Spain involving inoculating legume with acid tolerant rhizobia (Rodriguez-Echeverria and Perez-Fernandez, 2005). Local studies have also shown the benefit of utilizing acid tolerant rhizobia for improving crop productivity in acid soil under field and greenhouse conditions (Paterno et al.,1996; Delfin et al., 2003).
Low phosphorus availability is one of the common problems in acid soils. The strategies involved in increasing productivity under low P condition were increased use of P fertilizer and utilization of microbial-based technologies. Among the microbial-based technologies are inoculation with mycorrhiza and phosphate solubilizing bacteria. Mycorrhiza, specifically vesicular arbuscular mycorrhiza (VAM) enhanced the availability of P through enhanced uptake and translocation of phosphate ions from soil solution (Harley and Smith, 1983). On the other hand, phosphate solubilizing bacteria (PSB) solubilize insoluble P through organic acid production (Taha et al., 1969). Inoculation of tomato with VAM and PSB under low P soil increases plant growth, microbial biomass and alkaline phosphatase activity in the rhizosphere (Kim et al., 1998).
METHODOLOGY
Screening of acid tolerant isolates for promoting plant growth was conducted in the growth chambers. Solid based formulations of the selected isolates were prepared for screening. The isolates were initially grown in the acidified media used for their isolations. The cultures were then grown in the production medium for 48 hours. Sterilized solid based carrier consisting of a mixture of soil and charcoal were then inoculated with the cultures.
Twelve selected acid tolerant PGPB isolates which produced IAA and showed ACC deaminase activity were screened for plant growth promotion under gnotobiotic condition using eggplant (Solanum melongena L.) as test crop. The isolates consisted of seven nitrogen fixers (PGPB 56, 57, 60, 81, 82, 84, 85) and five phosphate solubilizers (PGPB 46, 49, 86, 87, 89). Sterile Magenta jars were prepared with acidic Luisiana clay as the growing medium. Simple Nutrient Addition Program (SNAPTM) solution was used as source of plant nutrients. The treatments also included an existing acid tolerant isolate (TCeRe9), and an uninoculated control, and were replicated four times.
A 1:10 sterile water suspension of the solid based preparations of the test isolates was used to inoculate seeds at the time of sowing and transplanting. Eggplant seeds were surface sterilized, sown in sterile vermiculite in aluminum trays and drenched with the inoculant suspension. When transplanting, the seedling roots were dipped in the inoculant suspension. Dry matter yield was measured four weeks after transplantation.
Field evaluation of selected acid tolerant PGPB: Field trials were established to evaluate the performance of selected acid tolerant PGPB strains 84 and 89 under field condition at the Cavinti Municipal Nursery, Cavinti, Laguna. Commercial eggplant variety ‘Violeta’ and hot pepper variety ‘Aruy-uy’ were used as test plants. Seeds of both crops were inoculated at sowing and reinoculated at time of pricking and transplanting by dipping the roots in 100g inoculant: 2Lwater suspension.
Soil samples from the area were collected and submitted for analysis. The results of the soil analyses are shown in Table 1 and were used as a basis for the rate of fertilizer applied in the trial. The experiment was a two-factorial arranged in RCBD with 3 replications. The two factors included in the trial were fertilizer rates: full recommended fertilizer rate and half recommended fertilizer rate; and inoculation: without inoculation, and inoculation with PGPB 84 and PGPB 89. The full rate used for hot pepper was 140-70-140 kg NPK per ha while the half rate was 70-35-70 kg NPK per ha. A small dose of fertilizer (0.5 g per plant for full rate and 0.25 g for half rate) was mixed with the soil medium placed in seedling trays for pricking of seedlings. When transplanting, plots with full fertilizer treatment were applied with complete fertilizer (14-14-14) equivalent to 70-70-70 NPK per ha while the plots with half fertilizer rate treatment were applied with complete fertilizer equivalent to 35-35-35 NPK per ha. The remaining fertilizer requirements are divided into 3WAT and 6WAT.
Similar treatments were initially applied to eggplant. However, an additional fertilizer was added to the trial at 3 months after transplantation to boost new leaf growth while premature leaf senescence was observed. Ten grams of complete fertilizer (14-14-14) was added to eggplants in the full fertilizer treatment while 5 grams in the half fertilizer treatment. The 10 g complete fertilizer added was equivalent to an additional 56 kg NPK for the full fertilizer treatment while the additional 5 g was equivalent to 28 kg NPK for the half fertilizer treatment. So far, only a few fruits were harvested from the area.
RESULTS AND DISCUSSION
Screening of acid tolerant isolates for plant growth promotion was conducted in the growth chambers. Seven (7) nitrogen fixers (PGPB 56, 57, 60, 81, 82, 84, 85) and five (5) phosphate solubilizers (PGPB 46, 49, 86, 87, 89) which produced IAA and showed ACC deaminase activity were screened for plant growth promotion under gnotobiotic condition using eggplant (Solanum melongena L.) as test crop (Figure 1). Figure 2 shows the effect of inoculation with the different isolates on the dry matter yield of eggplant at four weeks after transplantation. PGPB 84 and 89 gave the highest dry matter yields (Figure 3). Isolates PGPB 84 and PGPB 89 increased shoot dry matter yield by 14- and 16-fold, respectively, over the control. These isolates were further evaluated in the field.
Field evaluation of selected acid tolerant PGPB 84 and 89: Figures 4 and 5 show the response of hot pepper cv. Aruy-uy to inoculation with acid tolerant PGPB. Fruit yield of hot pepper was obtained from 4 plants in the inner rows, with an area measurement of 1 square meter. A total of 3 primings were obtained from the field trial due to the early onset of leaf spot diseases which resulted in early leaf senescence. Statistical analysis of yield data showed significant fertilizer rate-inoculation interaction. Significant response to PGPB 89 inoculation was obtained with full fertilizer rate application with the highest harvest of 207.50 g m-2 (Figure 6). Inoculation with PGPB 89 increased average total fruit weight by 105% and average total fruit number by 96% across fertilizer treatment. Interestingly, PGPB 84 inoculated plants performed poorly than uninoculated hot pepper in the fully fertilized plots. PGPB 89 inoculated plants produced the highest number of fruits averaged across fertilizer rates (Figure 7). Fertilizer rate did not have significant effect on the number of fruits formed.
Figures 8 and 9 show the response of eggplant cv. Violeta to inoculation with acid tolerant PGPB. Similar treatments were initially applied for eggplant. However, additional fertilizer was added to the trial at 3 months after transplanting to boost new leaf growth since premature leaf senescence was observed. Ten grams of complete fertilizer (14-14-14) were added to eggplant in the full fertilizer treatment while 5 grams was added to the half fertilizer treatment. The 10 g complete fertilizer added was equivalent to an additional 56 kg NPK for the full fertilizer treatment while the additional 5 g was equivalent to 28 kg NPK for the half fertilizer treatment. However, only a few fruits were harvested from the area.
A significant response to PGPB 89 inoculation by hot pepper was obtained in combination with the application of full fertilizer. PGPB 89 could produce both ACC deaminase and IAA. Growth promotion by PGPB has been attributed to phytohormone production such as IAA. The IAA enhances the rapid establishment of roots in young seedlings which increases their survival through increased nutrient and water uptake (Patten and Glick, 2002).
The acid-tolerant PGPBs isolates were deposited in the Philippine National Collection of Microorganisms (PNCM). Future studies include packaging of the inoculant formulation for testing in other acidic soil in the country and eventual registration to the Fertilizer and Pesticide Authority (FPA).
CONCLUSION
Acid tolerant bacteria were isolated, characterized and evaluated for growth promoting activities. Results showed that supplementing the recommended fertilizer rate with stress tolerant inoculants significantly increased yield of hot pepper and eggplant grown in acidic soils. Further greenhouse and field trials should provide more information on PGPB-plant variety interaction. Moreover, the stress tolerant PGPB inoculants should be further evaluated as components of nutrient management strategies to improve the productivity of degraded soils. The effect of the application of acid tolerant PGPB inoculants on the chemical and biological properties of Luisiana clay was assessed. Soil pH, organic matter content, exchangeable potassium and soil microbial biomass were not significantly affected by inoculation. In contrast inoculation significantly reduced exchangeable phosphorus. This may be related to an increase in plant uptake of P because of improved plant growth and increased microbial activities. The interaction between the introduced microorganism as inoculants and the indigenous soil microbial population is likely to be complex. Hence, a better understanding of the mechanisms of this interaction is necessary to predict short- and long-term effects (Bϋnemann, et al., 2006). The data obtained on the effect of PGPB inoculation on the environment are too limited to be able to draw a conclusion. A more extensive study on the effect of microbial inoculants on microbial diversity using molecular techniques should be the focus of future research.
ACKNOWLEDGEMENT
We are grateful for the funding support of the Department of Science and Technology- Philippine Council for Industry, Energy, and Emerging Technology Research and Development (DOST-PCIEERD) and the generosity of our farmer collaborators.
REFERENCES
Delfin, E.F., E.S. Paterno, A.M. Ocampo and F.C. Rodriguez.. 2003. Growth, yield and nutrient uptake of mungbean (vigna radiata l.) grown in Antipolo clay amended with lime, nitrogen and phosphorus fertilizers and microbial inoculants. The Phil. Agricultural Scientist 86: 75-83.
Dutta, S.K., Sarma, H.H., Saud, R.K., Konwar, M.J., Gogoi, B., Pathak, K., Bora, S.K., Mahanta, S., 2024. Assessment of organic and natural farming practices on quality parameters of joha rice and soil microbial biomass carbon (SMBC) in soil under cultivation. Plant Archives 24 (2), 2164–2168.
Harley JL, Smith SE. 1983 Mycorrhizal symbiosis. Academic Press, London, pp 317–356.
Kim, K.Y., D. Jordan and G.A. McDonald. 1998. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol Fertil Soils 26:79–87.
Marschner, H. 1991 Mechanisms of adaptation of plants to acid soils. Plant Soil 134, 1–20.
O’Callaghan, M., Ballard, R. A. & Wright, D. 2022. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use and Management, 38, 1340–1369. https://doi.org/10.1111/sum.12811
Paterno, E.S. M.L.Q. Sison, F.G.Torres, E.S. Garcia, S.M. Pantua and M.E. Orlina. 1996. Development of Microbial Inoculants for Stressed Environment. Terminal Report. BIOTECH,UPLB-PCASTRD Project.
Patten C. and Glick B.R., 1996. Bacterial biosynthesis of indole-3-acetic acid. Canadian Journal of Microbiology 42: 207-220.