DOI: https://doi.org/10.56669/QCOJ5580
ABSTRACT
Agricultural productivity is hampered by various biotic and abiotic stresses under varied crop ecosystems worldwide. Plant growth promoting rhizobacteria (PGPR) have emerged as effective tools for holistic crop health management. Bacteria are the most abundant microorganisms in soils compared to fungi and other microbes. Among PGPR, Bacillus species have been well recognized for its effectiveness against biotic and abiotic stresses, since it is most common bacteria found to easily colonize plants. They have been reported as plant growth promoter, inducer of systemic resistance, and used for production of a wide range of antimicrobial compounds (lipopeptides, antibiotics and enzymes) and competitors for growth factors (space and nutrients) with other phytopathogenic microorganisms through colonization. The prime aim of this chapter is to focus on niche areas in PGPR research with respect to Bacillus species, mechanism of action and their potential role in alleviation of biotic and abiotic stresses and growth promotion of crops.
Keywords: Plant growth promoting rhizobacteria, crop health, systemic resistance inducer, antimicrobial compounds, Bacillus, biotic and abiotic stresses
INTRODUCTION
Climate change poses serious threats due to abiotic and biotic stresses in plants which result in huge economic loss for farmers besides spoiling the food through production of toxins during storage. The conscious urge of farmers to combat the stresses lead to the development of a range of agrochemicals and their application which culminates in soil and groundwater contamination that ultimately endangers animal and human health. In order to get rid of these problems, biocontrol strategy for alleviation of plant diseases and environmental stresses assumed greater significance (Pal and Gardener 2006; Barr and Soila 1960).
The rhizosphere is densely inhabited with loads of microorganisms that are competing for space and nutrients (Walker et al. 2003). The soil microbiome is dynamic and affected by plant roots, soil management and other factors. Roots secrete primary and secondary metabolites, macromolecules and even cells into the rhizosphere that support nutrient acquisition and also shape the local microbiota (Driouich et al. 2013). Root exudates contain organic compounds that can serve as attractants for microorganisms which move towards roots using chemotaxis and may bring benefits to the plant (Ryan, Delhaize and Jones 2001). Exudates may also dissuade pathogens giving plants the possibility to affect the composition of the local soil microbiota (Baetz and Martinoia 2014). Further, certain microorganisms secrete compounds that favor their growth and association with plant roots.
Plant growth promoting rhizobacteria (PGPR) represent a wide group of bacteria that can colonize plant roots and support plant growth by mechanisms such as synthesis of phytohormones and increased nutrient uptake (Lugtenberg and Kamilova 2009). Many PGPR also have the capability to inhibit phytopathogens by releasing antibiotics (Ongena and Jacques 2008; Perez-Garcia et al. 2011) or by triggering (priming) innate immunity of plants referred to as induced systemic resistance (ISR) (Van Loon et al. 1998). It has been demonstrated that certain PGPR stimulate plant growth without being in physical contact with roots through release of volatile compounds (Ryu et al. 2003, 2004; Farag et al. 2006). Further, certain PGPR can restrict fungal growth by emission of volatile organic compounds (VOC) (Ryu et al. 2004; Ortiz- Castro et al. 2008). Bacterial VOC have been shown to serve various roles such as signal compounds for inter- and intra-species as well as cell-to-cell communication, stimulate or inhibit plant growth as well as affect phytopathogens (Wenke et al. 2010).
The use of agricultural chemical inputs can be reduced by soil microbes such as Bacillus sp. These beneficial bacterial strains are able to solubilize the immobile P in soil, which is then taken up by the plant roots (Ramani and Patel 2011; Tallapragada and Seshachala 2012). The biological health of soil remains poor if it has small amounts or nearly no microorganisms. It is considered as non-active soil and does not support healthy plant growth. Generally, the type and count of bacterial cells that are present in diverse soils are affected by soil environmental conditions such as pH, temperature, presence of various salts, heavy metals, moisture, and other inorganic and organic chemicals as well as by the types and number of flora and fauna found in that soils (Garbeva et al. 2004). The varied communities of aerobic endospore-forming beneficial bacteria (AEFB), i.e., Bacillus sp., commonly occur in all types of farming fields, with different types of plants, and play a significant role in enhancing crop productivity by its direct or indirect functions (Grayston et al. 1998). Other physiological properties, viz., multilayered cell walls, endospore formation which are stress resistant, and excretion of varied peptide antibiotics, signal peptide molecules, and extra cellular enzymes, are omnipresent in case of these various Bacillus sp., and these traits help in the proliferation and survival of bacterial cells under various adverse climatic conditions for very long duration (Pirttijärvi et al. 2000). Many species of Bacillus and Paenibacillus are very well recognized to augment plant development and growth. The chief means and approach for growth encouragement includes the manufacture of growth energizing phyto-hormones, solubilization and mobilization of insoluble phosphate present in soil, production of proteinaceous components such as siderophore, and demonstration of the phenomenon of antibiosis, i.e., antibiotics production. Besides these beneficial properties, several Bacilli are also involved in inhibition of plant ethylene production and stimulation of plant systemic resistance against several plant pathogens (Gutiérrez‐Mañero et al. 2008; Idris et al. 2004; 2007a, b; Richardson et al. 2009). The disease-inciting microbes negatively affect the plant growth and health and, therefore, are a major challenge to the production of food. Conventional approaches such as rotation of crops, breeding resistant plant varieties, and applying chemical pesticides seem to be inadequate for controlling plant root diseases of significant crops (Johri et al. 2003). Additionally, it seems unavoidable that lesser amounts of chemical pesticides will be employed and that more and more dependence will be rested on novel bioagents based microbial technology, which predominantly includes the application of antagonistic beneficial microbes as potential biopesticides. The research on diversity, characterization and applications of novel bioagents has increased recently, partly due to reform in public thought towards chemical residue free farming (Bale et al. 2008). There is also an urgent need to find suitable substitutes for harmful chemicals employed in plant disease control. There are several reports about the Bacillus and Paenibacillus species expressing antagonistic behaviors by the process of suppressing pathogens under in vivo and in vitro conditions (Govindasamy et al. 2010). Bacillus subtilis was first isolated in 1872 by Ferdinand Cohn which is a rod-shaped filament bacterium. Bacillus sp. are mainly focused on the frame of biological control due to their cosmopolitan distribution in diverse ecosystems, safety, combating ability against adverse environment (Earl et al. 2008; Nakkeeran et al. 2004, 2005; Montesinos and Bonaterra 2009). In the past few years, investigations have demonstrated that several Bacillus sp. like Bacillus subtilis GB03, B. amyloliquefaciens IN937a and Paenibacillus polymyxa E681 (Lee et al. 2012) secrete volatiles that stimulated growth of Arabidopsis thaliana. Bacillus subtilis GB03 emitted more than 25 volatiles that activated transcripts in A. thaliana involved in e.g. modification of cell walls, metabolism, hormone regulation and protein synthesis (Zhang et al. 2007). Additionally, B. subtilis GB03 volatiles regulated processes such as cell expansion, photosynthetic efficiency and seed set (Zhang et al. 2007; Xie, Zhang and Pare 2009). Several PGPR strains produce the volatiles 2R, 3R-butanediol and acetoin that trigger ISR as demonstrated for e.g. B. subtilis GB03 and B. amyloliquefaciens IN937a against Erwinia carotovora in A. thaliana (Ryu et al. 2004) and Pseudomonas chlororaphis O6 against E. carotovora in tobacco (Han et al. 2006). Bacterial VOC can have many different chemical structures where compounds such as amines, benzaldehyde, benzothiazole, decanal, cyclohexanol, dimethyl trisulfide, 2-ethyl-1-hexanol and nonanal have been identified as fungicidal molecules (Kai et al. 2009).
MINERAL SOLUBILIZATION
Phosphate solubilization
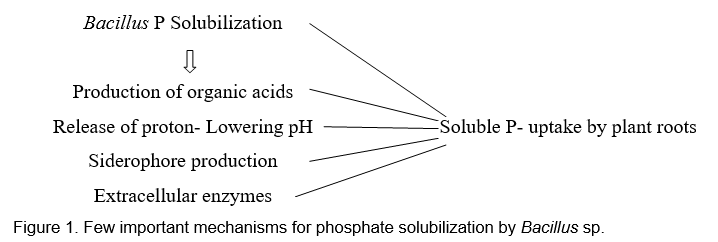
Minerals are naturally occurring inorganic chemical compound as a solid material and among 17 nutrients reported, Phosphorous plays a critical role in the plant growth through photosynthesis, energy transfer, transformation of sugars and starches and transformation of genetic materials from one generation to another. Acquisition of plant nutrients was enhanced by beneficial soil microorganisms. Insoluble forms of phosphatic fertilizer like tricalcium phosphate (Ca3PO4)2, aluminium phosphate (Al3PO4) and iron phosphate (Fe3PO4) were converted into available forms by microorganisms (Gupta et al. 2007; Song et al. 2008; Khan et al. 2013; Sharma et al. 2013). Wide range of biological process involved in the transformation of insoluble nutrients into soluble nutrients (Babalola and Glick, 2012). Two types of phosphate utilization were observed like direct application of phosphate fertilizer and microbial solubilization. In the soil, artificial application of phosphatic fertilizers leads to little amount of absorption by plants, and the remaining will be converted into insoluble complexes. These will be solubilized by microorganisms in higher-level conversion (Mckenzie and Roberts, 1990) mediated by the enzymes released by the soil microbes called phosphatases (Yadav and Tarafdar 2003; Tarafdar et al. 1988; Aseri et al. 2009) and phytases (Maougal et al. 2014). There are several reports of phosphate solubilisation by Bacillus sp. Kang et al. (2014) studied the beneficial aspects of B. megaterium strain mj1212 inoculated with mustard plants. It was observed that application of Bacillus sp. enhanced shoot and root length as well as plant fresh weight. The biochemical analysis showed that chlorophyll, fructose, glucose, sucrose, and many amino acids’ contents were higher in B. megaterium strain mj1212 inoculated plants, as compared to the uninoculated plants. Phosphate content was also higher in inoculated plants than control plants. In another study conducted by Swain et al. (2012), Bacillus subtilis thermotolerant strains (<50 °C) were isolated from cow dung. This strain also solubilized tricalcium phosphate, and this solubilization was associated with enzyme phosphatase production, especially the acid phosphatase (AcP). The inoculation of this bacterium with cowpea (Vigna unguiculata L.) resulted in increased root length, shoot height, and plant biomass as compared to the control plants. Wang et al. (2014) isolated phosphate-solubilizing bacteria Bacillus thuringiensis strain B1 from an acidic soil in China. Inoculation of B1 increased available P and peanut growth under acidic soil condition. Inoculation by strain B1 also considerably enhanced shoot length, branch number, hundred seed weight, and crude protein contents. Similar results on groundnut have also been reported by Maheswar and Sathiyavani (2012). As an endophytic bacterium, Bacillus sp. has been reported to solubilize P and enhance growth of banana plants (Matos et al. 2017). Kibrom et al. (2017) reported on the isolation and characterization of phosphate-solubilizing Bacillus sp. from various agroclimatic regions of Tigray, Ethiopia. The isolated bacteria enhanced P uptake as well as root and shoot length. Mohamed et al. (2018) isolated phosphate-solubilizing Bacillus subtilis and Serratia marcescens from the tomato plant rhizosphere. Inoculation of both bacteria in tomato plants enhanced phosphate uptake. Similarly, Turan et al. (2007) reported about the influence of Bacillus strain FS3 on growth and development of tomato (Lycopersicon esculentum L.) plants and enhanced phosphate content. The inoculation of FS3 increased plant height and root length. In another study conducted by Tahir et al. (2013), three phosphate-solubilizing bacterial strains, viz., Azospirillum, Bacillus, and Enterobacter, were isolated and characterized. Later these strains were identified based on 16SrRNA sequence analysis. Inoculation of these three strains improved wheat (Triticum aestivum L.) growth and phosphate uptake in grains. Jadhav (2016) studied the phosphate solubilization and biocontrol aspects of Bacillus licheniformis isolated from the pigeon pea (Cajanus cajan L.) rhizosphere. It was found that Bacillus licheniformis solubilized a good amount of phosphate under laboratory conditions. Owing to its P-solubilizing attribute, this isolate may be utilized as a potential P-mobilizing biofertilizer. Ahmad et al. (2018) studied the effect of phosphate-solubilizing Bacillus subtilis Q3 and Paenibacillus sp. Q6 for enhancing cotton plant growth under alkaline soil conditions. The strains Q3 and Q6 enhanced plant growth and P uptake. From a unique rhizosphere of an aromatic plant, Tallapragada and Seshachala (2012) isolated and characterized phosphate-solubilizing microbes from the rhizospheres of Piper betel. The isolated Bacillus sp. exhibited good amount of phosphate-solubilizing potential. Under semiarid conditions, the effect of salt-tolerant and phosphate solubilizers Bacillus sphaericus and Burkholderia cepacia were evaluated by Ramani and Patel (2011) on food and fodder crops. Both the bacterial isolates exhibited noteworthy effect under pot culture and field conditions. Bahadir et al. (2018) discovered potential bioinoculants 440 Bacillus isolates from various sources. These were investigated qualitatively for P solubilization, and affirmative isolates were further tested for quantitative determination of P solubilization and production of organic acid. The six best phosphate solubilizers were again tested for production of phytohormone (IAA), seed germination under in vitro conditions, and pot experiments. All six best Bacillus strains produced a good amount of IAA, meaningfully improved root and shoot length, and noticeably enhanced plant growth and development. In another study, the effect of P-solubilizing B. pumilus was studied on cauliflower. Bacillus sp. not only improved P uptake but also enhanced cauliflower size and weight as compared to the control (Dipta et al. 2017). The phosphate-solubilizing Bacillus sp. employed several mechanisms for P solubilization.
Potassium solubilization
Potassium (K) is considered as a major constituent and essential element in all living cells. Naturally, soils contain K in larger amounts than any other nutrients; however, most of the K is unavailable for plant uptake. Depending on soil type, 90–98% of potassium in the soil is in the unavailable form (Sparks and Huang, 1985). This can be converted to soluble forms by potassium-solubilizing bacteria for the plant uptake (Etesami et al. 2017) and mostly belong to the genera Bacillus sp. include B. pumilus, B. mucilaginous, B. amyloliquefaciens, B. firmus, B. megaterium, B. subtilis, B. licheniformis, Paenibacillus macerans (Glick 2012) having the capacity to solubilize K minerals like feldspar, muscovite, biotite, orthoclase, illite and mica. This can be made possible by various processes in conversion of silicate minerals through the process like acidolysis, complexolysis, chelation and exchange reaction. Upon artificial inoculation of phosphate-solubilizing bacteria may lead to improve plant growth by increasing seed emergence, plant weight and yield.
ALLEVIATION OF CROP STRESSES
Bacillus sp. is recognized as effective bioagent as well as plant growth promoting bacteria, and its potential has been proved during the last 20 years (Fiddaman and Rossall 1995; Sharga 1997; Campbell 1989). These Bacillus sp. mostly include B. subtilis (Mishagi and Donndelinger 1990), B. insolitus, B. pumilus (McInroy and Kloepper 1995), Paenibacillus polymyxa (Shishido et al. 1999), B. amyloliquefaciens (Reva et al. 2002), B. cereus (Pleban et al. 1997), B. megaterium (McInroy and Kloepper 1995), B. licheniformis and B. endophyticus from the inner tissues of cotton plant. Besides, the endospore-forming potential of the Bacillus sp. enhances their survival capability under diverse environmental conditions. Some strains of Bacillus species have the ability to intrude into the innermost plant tissues and play an essential role in plant growth promotion and protection against biotic and abiotic stresses. Bacillus sp. has been reported to produce diverse antibiotics with multifaceted mode of action that aids in the control of various phytopathogens (Kumar 1999; Asaka and Shoda 1996). The antibiotic compounds are of diverse types and structures, viz. aliphatic hydrocarbons, fatty acids, phenolics, and lipopeptides (Silo-suh et al. 1994; Sathyaprabha et al. 2010; Mihailovi et al. 2011; Sadashiva et al. 2010; Musthafa et al. 2012; Mora et al. 2011). Among the antibiotic compounds, antimicrobial peptides of short-chained amino acids namely bacillomycin, subtilin, iturins, mersacidin, surfactins, bacilysin, and fengycins exhibit a noteworthy performance in plant disease management (Mora et al. 2011; Chung et al. 2008; Rajesh Kumar et al. 2014; Ramarathnam et al. 2007; Ongena and Jacques 2007; Vinodkumar and Nakkeeran 2015). Major antimicrobial peptides produced by Bacillus sp. can be grouped into three categories, viz., iturins, surfactins, and fengycins/plipastatins. Iturin produced by B. subtilis, was effective against wide spectrum of fungal phytopathogens. A significant decrease in seed mycoflora was noticed in the seed loads that were tested with iturin A with concentrations of 50–100 ppm (Asaka and Shoda 1996). Treatment of corn seeds with iturin A @ 5 and 20 g/100 Kg showed a significant reduction in total microbial count was observed. B. subtilis strain RB14 that was capable of producing iturin B and surfactin that aided in suppressing damping-off disease of tomato (Asaka and Shoda 1996). In addition to disease control, B. subtilis strain BACT-O also promoted the plant growth and yield of cucumber (Utkhede et al. 1999). Liang et al. (2007) reported that seed priming with Bacillus polymixa increased the seedling height of safflower. Two types of chitinase enzymes are synthesized by B. amyloliquefaciens and can inhibit F. oxysporum growth (Wang et al. 2004). Chitinase enzymes with high chitinitic character are also produced by B. subtilis against various fungal pathogens (Chang et al. 2009). A chitinolytic bacterial strain (YC300), isolated from a compost sample from Republic of Korea, and produced an iturin-like compound. Later, this strain identified as Paenibacillus koreensis also provided a fair to good antifungal activities against Colletotrichum lagenarium, F. oxysporum and S. sclerotiorum, B. cinerea and R. solani (Chung et al. 2000). In an independent research, iturin A isolated from bacteria showed much stronger performance than surfactin against phytopathogens (Asaka and Shoda 1996). Three strains, B. cereus (L-07-01), B. subtilis (H-08-02), and Bacillus mycoides (S-07-01), exhibited significant antifungal activity against F. graminearum (Fernando et al. 2005; Ramarathnam et al. 2007). Soil application of Bacillus sp. was highly effective in the management of Fusarium wilt of carnation (Rajesh Kumar, 2014). B. subtilis, B. amyloliquefaciens, B. licheniformis, and B. cereus resulted in inhibiting the soil, air, and post-harvest plant diseases (Yoshida et al. 2002). Zwittermicin A is an aminopolyol compound produced by B. cereus and is also known to possess good inhibitory action against pathogenic fungi including oomycetes group of pathogens (Silo-Suh et al. 1998; Fernando et al. 2005). Delivering of talc-based consortia formulation comprising of B. subtilis (S2BC-2) + Burkholderia cepacia (TEPF-Sungal) reduced vascular wilt and corm rot of gladiolus under protected cultivation. Besides, increase in cormel and corm production and flowering was promoted upon the application (Shanmugam and Kanoujia, 2011). Kumar et al. (2014) reported the abiotic as well as biotic stress regulating potential of Bacillus sp. isolated from different rhizospheric zones of India. It was found that Bacillus sp. could control the growth of pathogenic fungal species such as Sclerotium rolfsii, Botrytis ricini, Macrophomina phaseolina, Fusarium oxysporum, and Rhizoctonia solani. On the other hand, this bacterium also demonstrated abiotic stress tolerance against salinity (NaCl 7%), higher temperature (50 °C), and drought (−1.2 MPa). Zwittermicin A is also popularly used as a broad spectrum of compound against different harmful microbes (Silo-suh et al. 1998). These groups of compounds also exhibit diverse biological activity against Oomycetous plant diseases as well as insecticidal activity (Emmert et al. 2004). Moreover, there are several reports which demonstrated the biological activity against different groups of plant pathogens by Bacillus species (Kloepper et al. 2004; Correa et al. 2009; Jogaiah et al. 2010). B. amyloliquefaciens with 23 diverse AMP genes effectively inhibited S. sclerotiorum which causes stem rot of carnation. Further, it significantly enhanced the plant growth and yield (Vinodkumar et al. 2015). In another study, the synergistic action of iturin and surfactin against Colletotrichum gloeosporioides was performed successfully (Kim et al. 2010). Surfactin like compounds isolated from B. subtilis (R14) and Bacillus circulans (Das et al. 2008; Fernandes et al. 2007) were found to suppress multidrug-resistant bacteria such as Escherichia coli, Alcaligenes faecalis, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, and methicillin-resistant. The four strains of B. subtilis which were effective against cucurbit powdery mildew also exhibited highest antibacterial activity against Xanthomonas campestris pv. cucurbitae and Pectobacterium carotovorum sub-sp. Carotovorum (Romero et al. 2007). These strains produced lipopeptide antibiotics, viz. fengycins, surfactins, and iturins. Further, thin-layer chromatography studies and direct bioautography revealed that the antibacterial activity was correlated due to iturin compound. This result was further validated using defective mutants of lipopeptide, thereby elucidated the importance of AMPs in plant disease control. US EPA (Environmental Protection Agency) has also provided a catalogue of biopesticides in which commercial formulations of distinct strains of Bacillus that can be employed as biocontrol agents are prescribed (McSpadden Gardener, 2004). These products are accessible in distinct types of preparations such as a wettable powder, dry cakes, or liquid or suspension in a liquid, as it depends on the nature of relationship between the biocontrol strain and carrier molecule. Products such as Taegro, Sublilex, Companion, Serenade, and Kodiak are all dependent on the utilization of various strains of B. subtilis as a biocontrol agent. Kodiak is known for the eradication of root inhabiting disease-causing agents of soybean and cotton like Aspergillus sp., Alternaria sp., Fusarium sp., and R. solani. Moreover, Serenade (Agra Quest, Daius CA, USA) constituting B. subtilis strain QST713 is known to remediate the Cercospora leaf spot, early and late blight diseases related with different crop plants. Similarly, bacterial strain, Bacillus sp. AZ-11 was also found as effective against reduction in Ganoderma growth. Several species of the genus Bacillus have been reported as potential BCAs. For instance, the bacterium B. tequilensis is reported to manage root knot nematode when co-inoculated with Trichoderma harzianum (Tiwari et al., 2017). Thus, this bacterium can have dual advantage in irrigated pockets where several vegetables suffer from root knot nematode. Incidentally, B. tequilensis is also known to solubilize Zinc for increased yield of wheat and soybean (Khande et al., 2017). In arid region, one strain of B. firmus has also been reported as specific antagonist to Macrophomina phaseolina (Lodha et al. 2013) in earlier studies. Bajoria et al. (2008) isolated B. subtilis, B. cereus, B. pumilus and B. sphaericus from hot arid region having biocontrol potential against plant pathogenic fungi. Therefore, the isolated antagonistic bacterial strains will be potentially very useful and yield effective results in further field studies for management of this terrific pathogen.
PLANT GROWTH PROMOTION
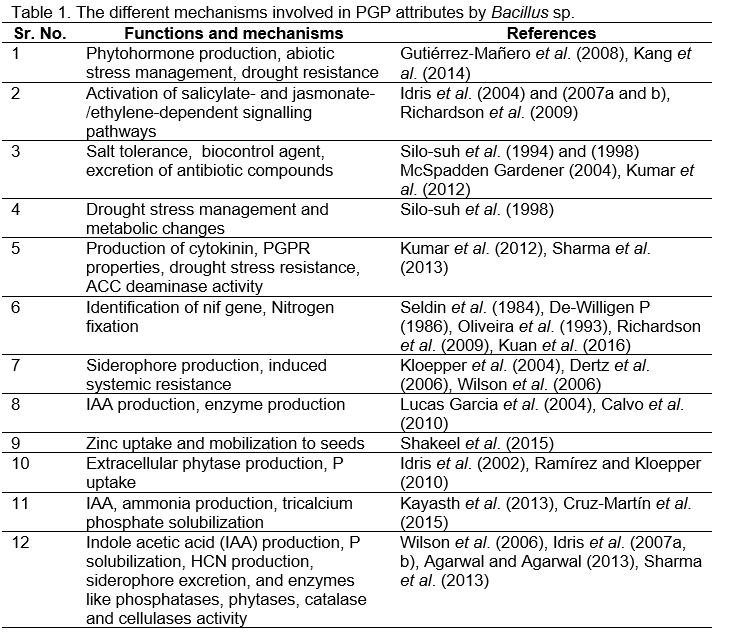
Bacillus sp. utilizes various means (direct as well as indirect) to promote plant growth. Bacillus sp. transforms the intricate or difficult form of indispensable nutrients, such as N and P, into very simple and bioavailable forms that can be taken up by the plant root system (Kuan et al. 2016; Shafi et al. 2017). The biological available form of Nitrogen in soils which is an essential constituent in nucleic acids, proteins, and other organic components, is partial or inadequate, which slows down plant growth in its natural ecosystem (Barker et al. 1974; De-Willigen 1986). There are some species of Bacillus sp. which release NH3 from nitrogenous organic matter (Hayat et al. 2010). Oliveira et al. (1993) reported that some of the Bacillus sp. possess the nif gene and demonstrate nitrogenase enzyme activity. This enzyme helps in the fixation of atmospheric Nitrogen and delivers it to the plants to augment plant development, growth, and yield by fulfilling N requirements and delaying the process of senescence (Seldin et al. 1984; Ding et al. 2005). The Bacillus sp. also excretes the proteinaceous compound known as a siderophore, which helps in iron chelation from the rhizospheric zone of plant (Wilson et al. 2006). The iron-chelating compound siderophores bind Fe+++ in complex substances and reduce the ferric form into ferrous form, which can easily enter plant root system (Dertz et al. 2006). Kumar et al. (2012) isolated seven soil bacterial isolates from bean rhizosphere in the Uttarakhand Himalayan region. These demonstrated excellent plant growth-promoting and biocontrol activities. On the bases of 16S rRNA gene sequence, the soil isolate was identified as Bacillus sp. and named BPR7. The rhizospheric strain BPR7 released phytohormone IAA, siderophores, enzyme phytase, organic acids, cyanogens, and ACC deaminase and was also able to solubilize numerous types of inorganic and organic phosphatic material as well as Zn and K. Gutiérrez-Mañero et al. (2008), reported that, B. pumilus and B. licheniformis isolated from alder (Alnus glutinosa [L.] Gaertn.) rhizosphere exhibited strong plant growth-promoting potential. Therefore, both the Bacillus bacteria demonstrated the good potential of promoting plant growth. The bioassay facts displayed that the dwarf phenotype produced in alder seedlings by a chemical paclobutrazol (which inhibits biosynthesis of gibberellin or GA) was reversed effectively by applying bacterial extracts from media and by exogenous treatment of GA3.
Calvo et al. (2010) isolated 63 Bacillus strains from the native potato rhizospheric regions growing in the Andean highlands of Peru. The strains demonstrated strong antagonism against Rhizoctonia solani and Fusarium solani. The antagonistic Bacillus strains were further verified for additional plant growth promotional aspects. It was observed that 81% of the isolates produced a certain amount of phytohormone indole-3-acetic acid (IAA) and 58% could solubilize tricalcium phosphate (TCP). The phylogenetic assessment revealed that the majority of isolates belonged to B. amyloliquefaciens sp., while other three potential strains may be novel putative Bacillus sp. Therefore, these Bacillus sp. may be used as potential potato growth promoters. Shakeel et al. (2015) obtained 234 rhizospheric isolates from basmati super rice and basmati-385 varieties cultivated in clay loam and saline soils from various locations of Punjab province in Pakistan. Out of the total 234 rice rhizospheric isolates, 27 isolates solubilized Zinc (Zn) from zinc oxide, zinc carbonate, and zinc phosphate. The soil isolate SH10 exhibited maximum zone of Zn solubilization (24 mm) using zinc phosphate, and soil isolate SH17 showed maximum zone of Zn solubilization (14–15 mm) using zinc oxide and zinc carbonate. Strains SH10 and SH17 also solubilized P (38–46 mm) and K (47–55 mm) under laboratory conditions. Both the strains also exhibited bio control activities against root rot (Fusarium moniliforme) and blast (Pyricularia oryzae) inciting plant pathogens by 22–29% and manufactured many biological control factors in vitro conditions. Bacterial strains SH10 and SH17 also enhanced Zinc uptake and translocation into the grains and augmented plant yield of super basmati rice and basmati-385 varieties by 18–47% and 22–49%, correspondingly. Using 16S rRNA gene analysis, both the SH10 and SH17 bacterial strains were identified as Bacillus sp. and Bacillus cereus. Idris et al. (2002) isolated many Bacillus strains belonging to B. subtilis or B. amyloliquefaciens from plant pathogen-contaminated soils and demonstrated PGPR properties. Soil isolate B. amyloliquefaciens could biodegrade extracellular compound phytate (myoinositol hexakisphosphate). The maximum phytase activity was found in isolate FZB45 (B. amyloliquefaciens), and the diluted bacterial cultural filtrate of this isolate encouraged maize seedling growth in phytate presence and P limitation. Lucas Garcia et al. (2004) studied the effect of inoculation of B. licheniformis on development and growth of tomato and pepper in three experiments. In the first experimental condition, the bacterium meaningfully enhanced plant height and the leaf surface area in both cultivars, and the effect was more on pepper as compared to tomato. In a second experiment, the plant seedlings were grown in sand, and hydroponic systems were tested. Interestingly, the size and number of tomato fruit increased after application of bacterial inoculation in sand and in the hydroponic system. Moreover, the inoculated cultivars exhibited less disease infestation as compared to untreated plants. In the third experiment, the pepper yield was greater in inoculated plants as compared to the uninoculated plants. This Bacillus strain had appreciable colonization and survival potential, and it could be employed as a biofertilizer as well as biocontrol agent. Kayasth et al. (2013) isolated Bacillus strain and designated as ML3 from a soil sample, and it produced the phytohormone and IAA and NH3 in the range of 174.72 and 0.66 μg ml−1, correspondingly. This strain also produced siderophores and effectively solubilized tricalcium phosphate (TCP). The main functional nitrogenase gene nif H was also found in this strain. Based on morphological, biochemical, and partial 16S rRNA gene sequencing, this strain ML3 was identified as B. licheniformis. This strain has all properties to be utilized as an efficient plant growth promoter. Agarwal and Agarwal (2013) isolated 28 bacterial strains from dissimilar tomato rhizospheric soils from Dehradun area of Uttarakhand, India. All of the soil isolates were characterized biochemically and tested for plant growth-promoting abilities such as indole acetic acid production (IAA), Phosphate (P) solubilization, HCN production, siderophore excretion, and catalase activity. Out of 28 soil isolates, only 5 Bacillus isolates exhibited potential plant growth-promoting abilities. Inoculation of tomato plants with selected Bacillus sp. resulted in increment in shoot and root length of tomato seedlings as compared to the uninoculated plants. Bacillus sp. also enhanced the seed germination percentage as well as seed vigor index. Ramírez and Kloepper (2010) have studied the consequence of inoculum concentration and soil phosphate-associated properties on plant growth encouragement by B. amyloliquefaciens strain FZB45, which produced phytase. A noteworthy synergy between soil phosphate and bacterial application was observed. B. amyloliquefaciens strain FZB45 encouraged plant growth and phosphate uptake, which confirms the role of enzyme phytase and less P uptake in uninoculated plants. This strain also produced IAA under laboratory conditions, but its role was not determined. Sharma et al. (2013) isolated a bacterium from the rhizosphere of soybean plants grown at Directorate of Soybean Research, Indore, Madhya Pradesh, India, and this bacterium was identified as Bacillus sp. on the basis of morphological and biochemical tests as well as FAME profile. Studies on 16S rRNA gene showed 98.7% homology to B. amyloliquefaciens, and thereafter, it was designated as strain sks-bnj-1 (AY 932823). This strain owned manifold plant growth-promoting attributes such as IAA production; siderophore production; ACC deaminase activity; enzymes like phosphatases, phytases, and cellulases; Zinc solubilization; and HCN production. This bacterium also exhibited biocontrol properties. Interaction of this strain with soybean increased the shoot root biomass as well as nutrient uptake as compared to the uninoculated control plants. In another study on Bacillus sp. by Cruz-Martín et al. (2015) conducted on banana plant in Cuba, it was observed that strain B. pumilus could fix atmospheric nitrogen and was able to grow in nitrogen-free culture media and produced IAA (28.9 μg ml−1). Moreover, strain B. pumilus significantly enhanced the plant height and thickness of the stem, altered root architecture, and improved fresh and dry plant weight. In the recent past, a novel bacterium, B. firmus was isolated from naturally heated cruciferous residue amended soil that showed antagonistic activity against M. phaseolina. In dual culture tests, it produced a scarlet pigmentation only against M. phaseolina (Lodha et al., 2013). Separate dual culture tests were also performed to ascertain the activity of antagonistic bacterium against prevalent soil fungi, Fusarium oxysporum f. sp. cumini and a bio-agent T. harzianum. However, B. firmus could not inhibit the growth of any of these fungi nor any scarlet pigmentation was observed during interaction. Bacillus sp. is particularly suited for studies on biological control due to its omnipresence in soils, high thermal tolerance, rapid growth in broth culture and ready formation of resistant spores. They are uniformly distributed throughout the soil rather than being concentrated in the plant rhizosphere. The main characteristics of B. firmus includes its thermal tolerance (45°C), phosphate solubilizing nature, compatibility with T. harzianum, increased nodulation, plant growth promotion and potential to colonize roots.
BIO FORMULATIONS FOR DISEASE SUPPRESSION
Many locally available on-farm wastes were evaluated to select food substrates that can improve the shelf life of B. firmus for a reasonable period. Our efforts successfully culminated in identifying a suitable food substrate, which was combined with carrier to retain adequate moisture in the bio-formulated product so that bacterium could survive for a period of 180 days. The product designated as Maru Sena 3 was made available for distribution to the farmers. The technology was validated after developing bio-formulated products and their efficacy was demonstrated at growers’ field to disseminate this eco-friendly and easy management strategy in the region (Mawar et al., 2018). Field demonstrations were carried out at adopted villages of the Institute’s Krishi Vigyan Kendra (Farm Science Centre dedicated for extension activities). Effectiveness of seed coating with bio-formulated product of B. firmus on incidence of dry root rot and seed yield of cluster bean, moth bean and sesame were demonstrated at grower’s field during last ten years. The percent increase in seed yield just by seed treatment ranged from 13.3-23.5% in all legumes and oil seed crops in different demonstrations. These products are made available for the farmers through Agricultural Technology Information Center (ATIC: Single window delivery mechanism) of the institute. In the last five years, hundreds of hectares have been sown with bacterium coated seeds. Farmers are getting positive response in checking incidence of dry root rot in rainfed crops of arid region namely cluster bean, cowpea, green gram, mothbean, sesame, etc. The process of developing bio-formulated product of these bio-pesticides has been patented in order to put these products for commercialization for wider adoption among growers. A patent “Bioformulation of a biopesticide and a process for preparing the same” was granted in 2019 with patent no. 309385.
Various studies reported that certain biocontrol consortia were unable to show at least comparable effects on plants when compared to their individual applications. One of the major causes for such contrary results may be attributed to incompatibility of the microbes in the mixture with each other. The findings clearly advocate for screening of compatible microbes for development of microbial consortia. The basic objective of developing microbial consortium would fail if the microbes used in the consortium do not have any additive or synergistic effects on disease suppression.
Application of bioagents in consortium may improve efficacy, reliability and consistency of the microbes under diverse soil and environmental conditions (Berg et al., 2005). Use of different species of biocontrol occupy different niches in the root zone and thereby restrict competition among them. Diversity in biocontrol mechanisms offered by each microbial component may also help in enhancing disease suppressiveness. A number of microbial consortia have been developed by many scientists and were tested in different crops in our country. Therefore, in the same stream our consortia prepared with two organisms worked in a synergistic manner. B. firmus operates through antibiosis while T. harzianum kills the mycelium of the pathogen mainly through hyper parasitism. It was, therefore, thought worthwhile to develop a consortium of native bio-control agents in the form of a single bio-formulated product to improve pathogen control by way of operating different mechanisms of antagonism for plant disease suppression. The biocontrol consortium activates the antioxidant enzyme activities and the phenylpropanoid pathway leading to accumulation of total phenolics, proline and pathogenesis related (PR) proteins after the pathogen challenge. The impact of triple microbial consortia consisting of fluorescent Pseudomonas (PHU094), Trichoderma (THU0816) and Rhizobium (RL091) for alleviation of biotic stress in chickpea is through enhanced antioxidant and phenylpropanoid activities (Akanksha et al., 2012). Periodical oxidase and accumulation of total phenol content was higher when challenged with the pathogen compared to the single microbe and dual microbial consortia. Pseuomonas aeruginosa PJHU15, Tricoderma harzianum TNHU27 and Bacillus subtilis BHHU100 as a consortium has been used to assess suppression of soft-rot pathogen Sclerotina sclerotiorum (Jain et al., 2012). The triple-microbe consortium and single-microbe treatments showed 1.4-2.3 and 1.1-1.7 fold increment in defense parameters respectively when compared to untreated challenged control. The compatible microbial consortia triggered defense responses in an enhanced level in pea than when the microbes were alone and provided better protection against Sclerotinia rot. Trichoderma species and fluorescent Pseudomonas sp. have been reported to induce systemic resistance in plants. These biological control agents were tested as a single application and in combination for their abilities to elicit induced resistance in cucumber against Fusarium oxysporum f. sp. radices cucumerinum and in A. thaliana against Botrytis cinerea. The combination of Tr6 and Ps14 induced a significantly higher level of resistance in cucumber, which was associated with the primed expression of a set of defense-related genes upon challenge with Fusarium. In Arabidopsis, both Ps14 and Tr6 triggered ISR against B. cinerea but their combination did not show enhanced effects. In the induced systemic resistance-defective Arabidopsis mutant myb72, none of the treatments protected against B. cinerea, whereas in the SA-impaired mutant sid2, all treatments were effective. Taken together, these results indicate that in Arabidopsis Ps14 and Tr6 activate the same signaling pathway and thus have no enhanced effect in combination. The enhanced protection in cucumber by the combination is most likely due to activation of different signaling pathways by the two biocontrol agents.
CONCLUSION AND PERSPECTIVES
Diversity in Bacillus species produce multiple classes of antimicrobial compounds inducing systemic resistance in a variety of ways that can be used for the plant growth promotion and management of a broad range of plant stresses and ultimately sustaining crop health. The spore forming ability of Bacillus species gives them a key importance in the field of biological control. The research areas that demand considerations for the successful adoption of Bacillus sp. as bioagents comprise the exploration for biodiverse antagonistic and antibiotic strains, elucidation of their mode of action and signalling pathways, stability under field application, development of effective formulations that can be used with other bioinoculants with synergetic effect and perfect demonstration of cost–benefit ratio for effective commercialization.
REFERENCES
Agrawal DP, Agrawal S (2013) Characterization of Bacillus sp. strains isolated from rhizosphere of tomato plants (Lycopersicon esculentum) for their use as potential plant growth promoting rhizobacteria. Int J Curr Microbiol Appl Sci 2(10):406–417
Ahmad M, Ahmad I, Hilger TH, Nadeem SM, Akhtar MF, Jamil M, Hussain A, Zahi ZA (2018) Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. Peer J 6:e5122.
Asaka O, Shoda M (1996) Biocontrol of Rhizoctonia solani damping off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol 62:4081–4085
Aseri GK, Jain N, Tarafdar JC (2009) Hydrolysis of organic phosphate forms by phosphatases and phytase producing fungi of arid and semi-arid soils of India. Am-Eurasian J Agric Environ Sci 5:564–570
Babalola OO, Glick BR (2012) Indigenous African agriculture and plant associated microbes: current practice and future transgenic prospects. Sci Res Essays 7:2431–2439
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19: 90–8
Bahadir PS, Liaqat F, Eltem R (2018) Plant growth promoting properties of phosphate solubilising Bacillus species isolated from the Aegean Region of Turkey. Turk J Bot 42:183–196
Bale JS, van Lenteren JC, Bigler F (2008) Biological control and sustainable food production. Philos Trans R Soc Lond B Biol Sci 363(1492):761–776
Barker AV, Maynard DN, Mills HA (1974) Variations in nitrate accumulation among spinach cultivars. J Am Soc Horticult Sci 99:32–134
Barr PO, Soila P (1960) Introduction of soft cannula into artery by direct percutaneous puncture. Observations on technique and cannula materials. Angiology 11:168–172
Calvo P, Ormeño-Orrillo E, Martínez-Romero E, Zúñiga D (2010) Characterization of Bacillus isolates of potato rhizosphere from Andean soils of Peru and their potential PGPR characteristics. Braz J Microbiol 41(4):899–906
Campbell R (1989) Biological control of microbial plant pathogens. Cambridge University Press, Cambridge, MA, pp 218
Chang WT, Chen ML, Wang SL (2009) An antifungal chitinase produced by Bacillus subtilis using chitin waste as a carbon source. World J Microbiol Biotechnol 26(5):945–950
Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim SD, Roberts DP (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soil borne pathogens of cucumber and pepper. Appl Microbiol Biotechnol 80:115–123
Chung YR, Kim CH, Hwang I, Chun J (2000) Paenibacillus koreensis sp. nov., a new species that produces an iturin-like antifungal compound. Int J Sys Evol Microbiol 50:1495–1500
Correa OS, Montecchina MS, Berti MF, Fernandez FMC, Pucheu NL, Kerber NL, Garcia AF (2009) Bacillus amyloliquefaciens BNM122 a potential microbial biocontrol agent applied on soybean seeds, cause a minor impact on rhizosphere and soil microbial communities. Appl Soil Ecol 41:185–194
Cruz-Martín M, Mena E, Sánchez-García C, Roque B, Acosta-Suárez M, Pichardo T, Leiva-Mora M, Alvarado-Capó Y (2015) The effects of plant growth promoting Bacillus pumilus CCIBPC5 on ‘Grande naine’ (Musa AAA) plants in acclimatization stage. Biotecnología Vegetal 15(3):151–156
Das P, Mukherjee S, Sen R (2008) Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J App Microbiol 104(6):1675–1684
Dertz EA, Stintzi A, Raymond KN (2006) Siderophore-mediated iron transport in Bacillus subtilis and Corynebacterium glutamicum. J Biol Inorg Chem 11(8):1087–1097
De-Willigen P (1986) Supply of soil nitrogen to the plant during the growing season. In: Lambers H, Neeteson JJ, Stulen I (eds) Fundamental, ecological and agricultural aspects of nitrogen metabolism in higher plants. Martinus Nijhoff, Dordrecht, pp 417–432
Ding Y, Wang J, Liu Y, Chen S (2005) Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J Appl Microbiol 99(5):1271–1281
Dipta B, Kirti S, Bhardwaj S, Gupta S, Kaushal R (2017) Phosphate solubilizing potential of Bacillus pumilus for the enhancement of cauliflower (Brassica oleracea var. botrytis l.). EM Int 23(3):1541–1548
Driouich A, Follet-Gueye ML, Vicr ´e-Gibouin M (2013) Root border cells and secretions as critical elements in plant host defense. Curr Opin Plant Biol 16:489–95
Earl AM, Losick R, Kolter R (2s008) Ecology and genomics of Bacillus subtilis. Trends Microbiol 16:269–275
Etesami H, Emami S, Alikhani H (2017) Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects-a review. J Soil Sci Plant Nutr 17
Farag MA, Ryu C-M, Sumner LW (2006) GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67:2262–8
Fernandes PAV, Arruda IRD, Santos AFABD, Araújo AAD, Maior AMS, Ximenes EA (2007) Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz J Microbiol 38(4):704–709
Fernando WGD, Ramarathnam R, Krishnamoorthy AS (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Fiddaman PJ, Rossall S (1995) Selection of bacterial antagonists for the biological control of Rhizoctonia solani in oilseed rape (Brassica napus). Plant Pathol 44:695–703
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Ann Rev Phytopathol 42:243–270
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica
Govindasamy V, Senthilkumar M, Magheshwaran V, Kumar U, Bose P, Sharma V, Annapurna K (2010) Bacillus and Paenibacillus sp.: potential PGPR for sustainable agriculture. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin/Heidelberg, pp 333–356
Grayston SJ, Wang S, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Gupta N, Sabat J, Parida R, Kerkatta D (2007) Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot Croat 66:197–204
Gutiérrez‐Mañero FJ, Ramos B, Probanza A, Mehouachi J, Talon M (2008) The plant growth promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberelins. Physiol Plant 111:206–211
Han SH, Lee SJ, Moon JH (2006) GacS-dependent production of 2R, 3R-Butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant Microbe In 19:924–30
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Idris EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Richter T, Borriss R (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth- promoting effect. Microbiology 148:2097–2109
Idris EE, Bochow H, Ross H, Borriss R (2004) Use of Bacillus subtilis as biocontrol agent. VI. Phytohormone action of culture filtrate prepared from plant growth promoting Bacillus amyloliquefaciens FZB24, FZB42, FZB45 and Bacillus subtilis FZB37. J Plant Dis Prot 111:583–597
Idris EE, Iglesias DJ, Talon M (2007a) Tryptophan dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant-Microbe Interact 20(6):619–626
Idris EES, Iglesias DJ, Talon M, Borriss R (2007b) Tryptophan-dependent production of Indole-3- Acetic Acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact 20:619–626
Jadhav RN (2016) Study of phosphate solubilization and antimicrobial activity of Bacillus licheniformis isolated from rhizosphere of Cajanus cajan cultivated in Marathwada region. Life Sci Int Res J 3(2):129–132
Jogaiah S, Roopa KS, Pushpalatha HG, Shekar Shetty H (2010) Evaluation of plant growth-promoting rhizobacteria for their efficiency to promote growth and induce systemic resistance in pearl millet against downy mildew disease. Arch Phytopathol Plant Prot. 43:368– 378
Johri BN, Sharma A, Virdi JS (2003) Rhizobacterial diversity in India and its influence on soil and plant health. Adv Biochem Eng Biotechnol 84:49–89
Kai M, Haustein M, Molina F (2009) Bacterial volatiles and their action potential. Appl Microbiol Biot 81:1001–12
Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH (2014) Phosphate solubilising Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol 54(4):427–433
Kayasth M, Kumar V, Gera R (2013) Exploring the potential of PGPR strain Bacillus licheniformis to be developed as multifunctional biofertilizer. Cent Eur J Exp Biol 2(1):12–17
Khan MS, Ahmad E, Zaidi A, Oves M (2013) Functional aspect of phosphate-solubilizing bacteria: importance in crop production. In: Maheshwari DK (ed) Bacteria in agrobiology: crop productivity. Springer, Berlin, pp 237–265
Kibrom FG, Alemayehu W, Prakasam VR, Kiros W (2017) Isolation and characterization of efficient phosphate solubilizing Bacillus (PSB) from different agro-ecological zones of Tigray Soil, Ethiopia. Momona Ethiop J Sci 9(2):262–273
Kim PI, Ryu J, Kim YH, Chi YT (2010) Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20(1):138–145
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus sp. Phytopathology 94:1259–1266
Kuan KB, Othman R, Rahim KA, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 11:e0152478
Kumar BSD (1999) Fusarial wilt suppression and crop improvement through two rhizobacterial strains in chickpea growing in soils infested with Fusarium oxysporum f.sp. ciceris. Biol Fertil Soils 29:87–91
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167(8):493–499
Lee B, Farag MA, Park HB (2012) Induced resistance by a longchain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS One 7:e48744
Liang BH, Shi ZQ, Zhang T, Yang ZM (2007) Fengycin antibiotics isolated from Bacillus sp. FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol Lett 272:91–98
Lodha S, Mawar,R. Chakarbarty P K and Bhagvan Singh (2013). Managing Macrophomina phaseolina causing dry root rot of legumes by a native strain of Bacillus firmus. Indian phytopathology 66 (4) : 356-360
Lucas Garcia JA, Probanza A, Ramos B, Ruiz Palomino M, Gutiérrez Mañero FJ (2004) Effect of inoculation of Bacillus licheniformis on tomato and pepper. Agronomie 24:169–176
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63: 541–56
Maheswar NU, Sathiyavani G (2012) Solubilization of phosphate by Bacillus Sps, from groundnut rhizosphere (Arachis hypogaea L). J Chem Pharm Res 4(8):4007–4011
Maougal RT, Brauman A, Plassard C, Abadie J, Djekoun A, Drevon JJ (2014) Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. Eur J Soil Biol 62:8–14
Matos ADM, Gomes ICP, Nietsche S, Xavier AA, Gomes WS, Neto JADS, Pereira MCT (2017) Phosphate solubilization by endophytic bacteria isolated from banana trees. Anais da Academia Brasileira de Ciências 89(4):2945–2954
McInroy JA, Kloepper JW (1995) Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 173:337–342
McKenzie RH, Roberts TL (1990) Soil and fertilizer phosphorus update. In: Alberta soil science workshop proceedings of coast terrace inn Edmonton, AB 84 104 20–22 Feb
Mcspadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus species in agricultural systems. Phytopathology 94:1252–1258
Mihailovi V, Vukovi N, Nićiforovi N, Soluji S, Mladenovi M, Mascaron P, Stankovi MS (2011) Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L. J Med Plants Res 5(7):1164–1174
Mishagi IJ, Donndelinger CR (1990) Endophytic bacteria in symptom free cotton plants. Phytopathology 9:808–811
Mohamed EAH, Farag AG, Youssef SA (2018) Phosphate solubilization by Bacillus subtilis and Serratia marcescens isolated from tomato plant rhizosphere. J Environ Prot 9(3):266–277
Montesinos E, Bonaterra A (2009) Microbial pesticides. In: Schaechter M (ed) Encyclopedia of microbiology, 3rd edn. Elsevier, pp 110–120
Mora I, Cabrefiga J, Montesinos E (2011) Antimicrobial peptide genes in Bacillus strains from plant environments. Int Microbiol 14:213–223
Musthafa KS, Balamurugan K, Pandian SK, Ravi AV (2012) 2,5-Piperazinedione inhibits quorum sensing-dependent factor production in Pseudomonas aeruginosa PAO1. J Basic Microbiol 52 (6):679–686
Nakkeeran S, Kavitha K, Mathiyazhagan S, Fernando WGD, Chandrasekar G, Renukadevi P (2004) Induced systemic resistance and plant growth promotion by Pseudomonas chlororaphis strain PA-23 and Bacillus subtilis strain CBE4 against rhizome rot of turmeric (Curcuma longa L.). Can J Plant Pathol 26:417–418
Nakkeeran S, Renukadevi P, Marimuthu T (2005) Antagonistic potentiality of Trichoderma viride and assessment of its efficacy for the management of cotton root rot. Arch Phytopathol Plant Prot 38(3): 209–225
Oliveira SS, Seldin L, Bastos MC (1993) Identification of structural nitrogen-fixation (nif) genes in Bacillus polymyxa and Bacillus macerans. World J Microbiol Biotechnol 9(3):387–389
Ongena M, Jacques P (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–25
Ortiz-Castro R, Valencia-Cantero E, Lopez-Bucio J (2008) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3:263–5
Pal KK, Gardener BM (2006) Biological control of plant pathogens. Plant Health Instruct. 2:1117–1142
Perez-Garcia A, Romero D, de Vincente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotech 22: 187–93
Pirttijärvi TS, Andersson MA, Salkinoja-Salonen MS (2000) Properties of Bacillus cereus and other bacilli contaminating biomaterial-based industrial processes. Int J Food Microbiol 60(2–3):231–239
Pleban S, Chernin L, Chet I (1997) Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett Appl Microbiol 25:284–288
Rajesh Kumar P (2014) Role of antimicrobial peptide genes of PGPR and fungicides for the management of stem rot of and wilt complex of carnation under protected cultivation. M.Sc. thesis, Tamil Nadu Agricultural University, Coimbatore, India
Rajesh Kumar P, Adhipathi P, Nakkeeran S (2014) Antimicrobial peptide genes of PGPR for the management of Fusarium wilt of carnation protected cultivation. 44:54–58
Ramani V, Patel HH (2011) Phosphate solubilization by Bacillus sphaericus and Burkholderia cepacia in presence of pesticides. J Agric Technol 7(5):1331–1337
Ramarathnam R, Bo S, Chen Y, Fernando WGD, Xuenwen G, De Kievit T (2007) Molecular and biochemical detection of fengycin and bacillomycin D producing Bacillus sp. antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53:901–911
Ramírez CA, Kloepper JW (2010) Plant growth promotion by Bacillus amyloliquefaciens FZB45 depends on inoculum rate and P-related soil properties. Biol Fertil Soil 46(8): 835–844
Reva O, Smirnov VV, Petterson B, Priest FG (2002) Bacillus endophyticus sp. nov., isolated from the inner tissues of cotton plants (Gossypium sp.). Int J Syst Evol Microbiol 52:101–107
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Romero D, Vicente A, Rakotoaly RH, Dufour SE, Veening JW, Arrebola E, Cazorla FM, Kuipers O, Paquot M, Garcia AP (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis towards Podosphaera fusca. Mol Plant Microbe Interact 118(2):323–327
Ryan P, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Phys 52:527–60
Ryu CM, Farag MA, Hu CH (2003) Bacterial volatiles promote growth in Arabidopsis. P Natl Acad Sci USA 100:4927–32
Ryu CM, Farag MA, Hu CH (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134: 1017–26
Sadashiva CT, Sharanappa P, Rema shree B, Raghu AV, Udayan PS, Balachandran I (2010) Chemical composition and antimicrobial activity of the essential oil from bark of Pittosporum dasycaulon Miq. Adv Biol Res 4:301–304
Sathyaprabha G, Kumaravel S, Ruffina D, Praveenkumar P (2010) A comparative study on antioxidant, proximate analysis, antimicrobial activity and phytochemical analysis of Aloe vera and Cissus quadrangularis by GC-MS. J Pharm Res 3(12):2970–2973
Seldin L, Van Elsas JD, Penido EGC (1984) Bacillus azotofixans sp. nov. a nitrogen-fixing species from Brazilian soils and grass roots. Int J Syst Bacteriol 34(4):451–456
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnological Equip. https://doi.org/10.1080/13102818.2017.1286950
Shakeel M, Rais A, Hassan MN, Hafeez FY (2015) Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati Rice (Oryza sativa) varieties. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01286
Sharga BM (1997) Bacillus isolates as potential biocontrol agents against chocolate spot on faba beans. Can J Microbiol 43:915–924
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer plus 2:587
Sharma SK, Ramesh A, Johri BN (2013) Isolation and characterization of plant growth-promoting Bacillus amyloliquefaciens strain sks_bnj_1 and its influence on rhizosphere soil properties and nutrition of Soybean (Glycine max L. Merrill). J Virol Microbiol 2013:446006. https://doi. org/10.5171/2013.446006
Shishido M, Breuil C, Chanway CP (1999) Endophytic colonization of spruce by plant growth promoting rhizobacteria. FEMS Microbiol Ecol 29:191–196
Silo-suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J (1994) Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW 85. Appl Environ Microbiol 60:2023–2030
Silo-suh LA, Stab VE, Raffel SR, Handelsman J (1998) Target range of Zwittermicin A, an Aminopolyol antibiotic from Bacillus cereus. Curr Microbiol 37:6–11
Sparks DL, Huang PM (1985) Physical chemistry of soil potassium. In: Potassium in agriculture, pp 201–276
Swain MR, Laxminarayana K, Ray RC (2012) Phosphorus solubilization by thermotolerant Bacillus subtilis isolated from cow dung microflora. Agric Res 1(3):273–279
Tahir M, Mirza MS, Zaheer A, Dimitrov MR, Smidt H, Hameed S (2013) Isolation and identification of phosphate solubilizer Azospirillum, Bacillus and Enterobacter strains by 16SrRNA sequence analysis and their effect on growth of wheat (Triticum aestivum L.). Aust J Crop Sci 7(9):1284–1292
Tallapragada P, Seshachala U (2012) Phosphate-solubilizing microbes and their occurrence in the rhizospheres of Piper betel in Karnataka. India Turk J Biol 36:25–35
Tarafdar JC, Rao AV, Bala K (1988) Production of phosphatases by fungi isolated from desert soils. Folia Microbiol 33:453–457
Turan M, Ataoğlu N, Şahin F (2007) Effects of Bacillus FS-3 on growth of tomato (Lycopersicon esculentum L.) plants and availability of phosphorus in soil. Plant Soil Environ 53(2):58–64
Utkhede RS, Koch CA, Menzies JG (1999) Rhizobacterial growth and yield promotion of cucumber plants inoculated with Pythium aphanidermatum. Can J Plant Path 21:265–271
Van Loon L, Bakker P, Pieterse C (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–83
Vinodkumar S, Nakkeeran S (2015) Synergistic action of antimicrobial peptide (AMP) genes in Bacillus amyloliquefaciens for the management of stem rot of carnation. J Mycol Plant Pathol 45(4):229–234
VinodKumar S, Rajesh Kumar P, Senthilraja C, Nakkeeran S, Fernando WGD (2015) First report of Sclerotinia sclerotiorum causing stem rot of carnation (Dianthus caryophyllus) in India. Plant Dis 99(9):1280
Walker TS, Bais HP, Grotewold E (2003) Root exudation and rhizosphere biology. Plant Physiol 132: 44–51
Wang J, Liu J, Wang X (2004) Application of electrospray ionization mass spectrometry in rapid typing of fengycin homologues produced by Bacillus subtilis. Lett Appl Microbiol 39 (1): 98–102
Wang T, Liu MQ, Li HX (2014) Inoculation of phosphate solubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agric Scand B Soil Plant Sci 64(3):252–259
Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506
Wilson MK, Abergel RJ, Raymond KN, Arceneaux JE, Byers BR (2006) Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem Biophys Res Commun 348(1):320–325
Xie X, Zhang H, Pare PW (2009) Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03). Plant Signal Behav 4: 948–53
Yadav RS, Tarafdar JC (2003) Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol Biochem 35:1–7
Yoshida S, Shirata A, Hiradate S (2002) Ecological characteristics and biological control of mulberry anthracnose. Jpn Agric Res Q 36:89–95
Zhang H, Kim MS, Krishnamachari V (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–51
Berg G, Eberl L and Hartmann A. (2005) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7, 1673–1685.
Akanksha S, Sarma BK, Upadhyay RS and Singh HB. (2012). Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiological Research 168, 33-40.
Jain A, Singh S, Sarma B Kumar and Singh HB. (2012). Microbial consortium–mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. Journal of Applied Microbiology 112(3):537-50.
Mawar R, Tomer A S and Dheeraj Singh (2018). Demonstration of bio-formulated products of bio agents against disease incidence and seed yield of crops in Indian arid region. Indian Phytopathology 71:1-6
Mawar R, and Lodha S. 2017. Native Bio-agents: An integral component for managing soil borne plant pathogens in arid region. In: Microbial Antagonists: their role in biological control of plant diseases. Today &Tomorrow’s Printers and Publishers, New Delhi-110 002, India
Mawar, R., Ram, L., Sharma, D. and Jangid, K., 2021. Bacterial antagonists against Ganoderma lucidum the incitant of root rot of Indian Mesquite.Indian Phytopathology, pp.1-6.
Status and Prospects of Bacillus Species in Alleviating Stresses and Enhancing Growth for Improvement of Crop Health
DOI: https://doi.org/10.56669/QCOJ5580
ABSTRACT
Agricultural productivity is hampered by various biotic and abiotic stresses under varied crop ecosystems worldwide. Plant growth promoting rhizobacteria (PGPR) have emerged as effective tools for holistic crop health management. Bacteria are the most abundant microorganisms in soils compared to fungi and other microbes. Among PGPR, Bacillus species have been well recognized for its effectiveness against biotic and abiotic stresses, since it is most common bacteria found to easily colonize plants. They have been reported as plant growth promoter, inducer of systemic resistance, and used for production of a wide range of antimicrobial compounds (lipopeptides, antibiotics and enzymes) and competitors for growth factors (space and nutrients) with other phytopathogenic microorganisms through colonization. The prime aim of this chapter is to focus on niche areas in PGPR research with respect to Bacillus species, mechanism of action and their potential role in alleviation of biotic and abiotic stresses and growth promotion of crops.
Keywords: Plant growth promoting rhizobacteria, crop health, systemic resistance inducer, antimicrobial compounds, Bacillus, biotic and abiotic stresses
INTRODUCTION
Climate change poses serious threats due to abiotic and biotic stresses in plants which result in huge economic loss for farmers besides spoiling the food through production of toxins during storage. The conscious urge of farmers to combat the stresses lead to the development of a range of agrochemicals and their application which culminates in soil and groundwater contamination that ultimately endangers animal and human health. In order to get rid of these problems, biocontrol strategy for alleviation of plant diseases and environmental stresses assumed greater significance (Pal and Gardener 2006; Barr and Soila 1960).
The rhizosphere is densely inhabited with loads of microorganisms that are competing for space and nutrients (Walker et al. 2003). The soil microbiome is dynamic and affected by plant roots, soil management and other factors. Roots secrete primary and secondary metabolites, macromolecules and even cells into the rhizosphere that support nutrient acquisition and also shape the local microbiota (Driouich et al. 2013). Root exudates contain organic compounds that can serve as attractants for microorganisms which move towards roots using chemotaxis and may bring benefits to the plant (Ryan, Delhaize and Jones 2001). Exudates may also dissuade pathogens giving plants the possibility to affect the composition of the local soil microbiota (Baetz and Martinoia 2014). Further, certain microorganisms secrete compounds that favor their growth and association with plant roots.
Plant growth promoting rhizobacteria (PGPR) represent a wide group of bacteria that can colonize plant roots and support plant growth by mechanisms such as synthesis of phytohormones and increased nutrient uptake (Lugtenberg and Kamilova 2009). Many PGPR also have the capability to inhibit phytopathogens by releasing antibiotics (Ongena and Jacques 2008; Perez-Garcia et al. 2011) or by triggering (priming) innate immunity of plants referred to as induced systemic resistance (ISR) (Van Loon et al. 1998). It has been demonstrated that certain PGPR stimulate plant growth without being in physical contact with roots through release of volatile compounds (Ryu et al. 2003, 2004; Farag et al. 2006). Further, certain PGPR can restrict fungal growth by emission of volatile organic compounds (VOC) (Ryu et al. 2004; Ortiz- Castro et al. 2008). Bacterial VOC have been shown to serve various roles such as signal compounds for inter- and intra-species as well as cell-to-cell communication, stimulate or inhibit plant growth as well as affect phytopathogens (Wenke et al. 2010).
The use of agricultural chemical inputs can be reduced by soil microbes such as Bacillus sp. These beneficial bacterial strains are able to solubilize the immobile P in soil, which is then taken up by the plant roots (Ramani and Patel 2011; Tallapragada and Seshachala 2012). The biological health of soil remains poor if it has small amounts or nearly no microorganisms. It is considered as non-active soil and does not support healthy plant growth. Generally, the type and count of bacterial cells that are present in diverse soils are affected by soil environmental conditions such as pH, temperature, presence of various salts, heavy metals, moisture, and other inorganic and organic chemicals as well as by the types and number of flora and fauna found in that soils (Garbeva et al. 2004). The varied communities of aerobic endospore-forming beneficial bacteria (AEFB), i.e., Bacillus sp., commonly occur in all types of farming fields, with different types of plants, and play a significant role in enhancing crop productivity by its direct or indirect functions (Grayston et al. 1998). Other physiological properties, viz., multilayered cell walls, endospore formation which are stress resistant, and excretion of varied peptide antibiotics, signal peptide molecules, and extra cellular enzymes, are omnipresent in case of these various Bacillus sp., and these traits help in the proliferation and survival of bacterial cells under various adverse climatic conditions for very long duration (Pirttijärvi et al. 2000). Many species of Bacillus and Paenibacillus are very well recognized to augment plant development and growth. The chief means and approach for growth encouragement includes the manufacture of growth energizing phyto-hormones, solubilization and mobilization of insoluble phosphate present in soil, production of proteinaceous components such as siderophore, and demonstration of the phenomenon of antibiosis, i.e., antibiotics production. Besides these beneficial properties, several Bacilli are also involved in inhibition of plant ethylene production and stimulation of plant systemic resistance against several plant pathogens (Gutiérrez‐Mañero et al. 2008; Idris et al. 2004; 2007a, b; Richardson et al. 2009). The disease-inciting microbes negatively affect the plant growth and health and, therefore, are a major challenge to the production of food. Conventional approaches such as rotation of crops, breeding resistant plant varieties, and applying chemical pesticides seem to be inadequate for controlling plant root diseases of significant crops (Johri et al. 2003). Additionally, it seems unavoidable that lesser amounts of chemical pesticides will be employed and that more and more dependence will be rested on novel bioagents based microbial technology, which predominantly includes the application of antagonistic beneficial microbes as potential biopesticides. The research on diversity, characterization and applications of novel bioagents has increased recently, partly due to reform in public thought towards chemical residue free farming (Bale et al. 2008). There is also an urgent need to find suitable substitutes for harmful chemicals employed in plant disease control. There are several reports about the Bacillus and Paenibacillus species expressing antagonistic behaviors by the process of suppressing pathogens under in vivo and in vitro conditions (Govindasamy et al. 2010). Bacillus subtilis was first isolated in 1872 by Ferdinand Cohn which is a rod-shaped filament bacterium. Bacillus sp. are mainly focused on the frame of biological control due to their cosmopolitan distribution in diverse ecosystems, safety, combating ability against adverse environment (Earl et al. 2008; Nakkeeran et al. 2004, 2005; Montesinos and Bonaterra 2009). In the past few years, investigations have demonstrated that several Bacillus sp. like Bacillus subtilis GB03, B. amyloliquefaciens IN937a and Paenibacillus polymyxa E681 (Lee et al. 2012) secrete volatiles that stimulated growth of Arabidopsis thaliana. Bacillus subtilis GB03 emitted more than 25 volatiles that activated transcripts in A. thaliana involved in e.g. modification of cell walls, metabolism, hormone regulation and protein synthesis (Zhang et al. 2007). Additionally, B. subtilis GB03 volatiles regulated processes such as cell expansion, photosynthetic efficiency and seed set (Zhang et al. 2007; Xie, Zhang and Pare 2009). Several PGPR strains produce the volatiles 2R, 3R-butanediol and acetoin that trigger ISR as demonstrated for e.g. B. subtilis GB03 and B. amyloliquefaciens IN937a against Erwinia carotovora in A. thaliana (Ryu et al. 2004) and Pseudomonas chlororaphis O6 against E. carotovora in tobacco (Han et al. 2006). Bacterial VOC can have many different chemical structures where compounds such as amines, benzaldehyde, benzothiazole, decanal, cyclohexanol, dimethyl trisulfide, 2-ethyl-1-hexanol and nonanal have been identified as fungicidal molecules (Kai et al. 2009).
MINERAL SOLUBILIZATION
Phosphate solubilization
Minerals are naturally occurring inorganic chemical compound as a solid material and among 17 nutrients reported, Phosphorous plays a critical role in the plant growth through photosynthesis, energy transfer, transformation of sugars and starches and transformation of genetic materials from one generation to another. Acquisition of plant nutrients was enhanced by beneficial soil microorganisms. Insoluble forms of phosphatic fertilizer like tricalcium phosphate (Ca3PO4)2, aluminium phosphate (Al3PO4) and iron phosphate (Fe3PO4) were converted into available forms by microorganisms (Gupta et al. 2007; Song et al. 2008; Khan et al. 2013; Sharma et al. 2013). Wide range of biological process involved in the transformation of insoluble nutrients into soluble nutrients (Babalola and Glick, 2012). Two types of phosphate utilization were observed like direct application of phosphate fertilizer and microbial solubilization. In the soil, artificial application of phosphatic fertilizers leads to little amount of absorption by plants, and the remaining will be converted into insoluble complexes. These will be solubilized by microorganisms in higher-level conversion (Mckenzie and Roberts, 1990) mediated by the enzymes released by the soil microbes called phosphatases (Yadav and Tarafdar 2003; Tarafdar et al. 1988; Aseri et al. 2009) and phytases (Maougal et al. 2014). There are several reports of phosphate solubilisation by Bacillus sp. Kang et al. (2014) studied the beneficial aspects of B. megaterium strain mj1212 inoculated with mustard plants. It was observed that application of Bacillus sp. enhanced shoot and root length as well as plant fresh weight. The biochemical analysis showed that chlorophyll, fructose, glucose, sucrose, and many amino acids’ contents were higher in B. megaterium strain mj1212 inoculated plants, as compared to the uninoculated plants. Phosphate content was also higher in inoculated plants than control plants. In another study conducted by Swain et al. (2012), Bacillus subtilis thermotolerant strains (<50 °C) were isolated from cow dung. This strain also solubilized tricalcium phosphate, and this solubilization was associated with enzyme phosphatase production, especially the acid phosphatase (AcP). The inoculation of this bacterium with cowpea (Vigna unguiculata L.) resulted in increased root length, shoot height, and plant biomass as compared to the control plants. Wang et al. (2014) isolated phosphate-solubilizing bacteria Bacillus thuringiensis strain B1 from an acidic soil in China. Inoculation of B1 increased available P and peanut growth under acidic soil condition. Inoculation by strain B1 also considerably enhanced shoot length, branch number, hundred seed weight, and crude protein contents. Similar results on groundnut have also been reported by Maheswar and Sathiyavani (2012). As an endophytic bacterium, Bacillus sp. has been reported to solubilize P and enhance growth of banana plants (Matos et al. 2017). Kibrom et al. (2017) reported on the isolation and characterization of phosphate-solubilizing Bacillus sp. from various agroclimatic regions of Tigray, Ethiopia. The isolated bacteria enhanced P uptake as well as root and shoot length. Mohamed et al. (2018) isolated phosphate-solubilizing Bacillus subtilis and Serratia marcescens from the tomato plant rhizosphere. Inoculation of both bacteria in tomato plants enhanced phosphate uptake. Similarly, Turan et al. (2007) reported about the influence of Bacillus strain FS3 on growth and development of tomato (Lycopersicon esculentum L.) plants and enhanced phosphate content. The inoculation of FS3 increased plant height and root length. In another study conducted by Tahir et al. (2013), three phosphate-solubilizing bacterial strains, viz., Azospirillum, Bacillus, and Enterobacter, were isolated and characterized. Later these strains were identified based on 16SrRNA sequence analysis. Inoculation of these three strains improved wheat (Triticum aestivum L.) growth and phosphate uptake in grains. Jadhav (2016) studied the phosphate solubilization and biocontrol aspects of Bacillus licheniformis isolated from the pigeon pea (Cajanus cajan L.) rhizosphere. It was found that Bacillus licheniformis solubilized a good amount of phosphate under laboratory conditions. Owing to its P-solubilizing attribute, this isolate may be utilized as a potential P-mobilizing biofertilizer. Ahmad et al. (2018) studied the effect of phosphate-solubilizing Bacillus subtilis Q3 and Paenibacillus sp. Q6 for enhancing cotton plant growth under alkaline soil conditions. The strains Q3 and Q6 enhanced plant growth and P uptake. From a unique rhizosphere of an aromatic plant, Tallapragada and Seshachala (2012) isolated and characterized phosphate-solubilizing microbes from the rhizospheres of Piper betel. The isolated Bacillus sp. exhibited good amount of phosphate-solubilizing potential. Under semiarid conditions, the effect of salt-tolerant and phosphate solubilizers Bacillus sphaericus and Burkholderia cepacia were evaluated by Ramani and Patel (2011) on food and fodder crops. Both the bacterial isolates exhibited noteworthy effect under pot culture and field conditions. Bahadir et al. (2018) discovered potential bioinoculants 440 Bacillus isolates from various sources. These were investigated qualitatively for P solubilization, and affirmative isolates were further tested for quantitative determination of P solubilization and production of organic acid. The six best phosphate solubilizers were again tested for production of phytohormone (IAA), seed germination under in vitro conditions, and pot experiments. All six best Bacillus strains produced a good amount of IAA, meaningfully improved root and shoot length, and noticeably enhanced plant growth and development. In another study, the effect of P-solubilizing B. pumilus was studied on cauliflower. Bacillus sp. not only improved P uptake but also enhanced cauliflower size and weight as compared to the control (Dipta et al. 2017). The phosphate-solubilizing Bacillus sp. employed several mechanisms for P solubilization.
Potassium solubilization
Potassium (K) is considered as a major constituent and essential element in all living cells. Naturally, soils contain K in larger amounts than any other nutrients; however, most of the K is unavailable for plant uptake. Depending on soil type, 90–98% of potassium in the soil is in the unavailable form (Sparks and Huang, 1985). This can be converted to soluble forms by potassium-solubilizing bacteria for the plant uptake (Etesami et al. 2017) and mostly belong to the genera Bacillus sp. include B. pumilus, B. mucilaginous, B. amyloliquefaciens, B. firmus, B. megaterium, B. subtilis, B. licheniformis, Paenibacillus macerans (Glick 2012) having the capacity to solubilize K minerals like feldspar, muscovite, biotite, orthoclase, illite and mica. This can be made possible by various processes in conversion of silicate minerals through the process like acidolysis, complexolysis, chelation and exchange reaction. Upon artificial inoculation of phosphate-solubilizing bacteria may lead to improve plant growth by increasing seed emergence, plant weight and yield.
ALLEVIATION OF CROP STRESSES
Bacillus sp. is recognized as effective bioagent as well as plant growth promoting bacteria, and its potential has been proved during the last 20 years (Fiddaman and Rossall 1995; Sharga 1997; Campbell 1989). These Bacillus sp. mostly include B. subtilis (Mishagi and Donndelinger 1990), B. insolitus, B. pumilus (McInroy and Kloepper 1995), Paenibacillus polymyxa (Shishido et al. 1999), B. amyloliquefaciens (Reva et al. 2002), B. cereus (Pleban et al. 1997), B. megaterium (McInroy and Kloepper 1995), B. licheniformis and B. endophyticus from the inner tissues of cotton plant. Besides, the endospore-forming potential of the Bacillus sp. enhances their survival capability under diverse environmental conditions. Some strains of Bacillus species have the ability to intrude into the innermost plant tissues and play an essential role in plant growth promotion and protection against biotic and abiotic stresses. Bacillus sp. has been reported to produce diverse antibiotics with multifaceted mode of action that aids in the control of various phytopathogens (Kumar 1999; Asaka and Shoda 1996). The antibiotic compounds are of diverse types and structures, viz. aliphatic hydrocarbons, fatty acids, phenolics, and lipopeptides (Silo-suh et al. 1994; Sathyaprabha et al. 2010; Mihailovi et al. 2011; Sadashiva et al. 2010; Musthafa et al. 2012; Mora et al. 2011). Among the antibiotic compounds, antimicrobial peptides of short-chained amino acids namely bacillomycin, subtilin, iturins, mersacidin, surfactins, bacilysin, and fengycins exhibit a noteworthy performance in plant disease management (Mora et al. 2011; Chung et al. 2008; Rajesh Kumar et al. 2014; Ramarathnam et al. 2007; Ongena and Jacques 2007; Vinodkumar and Nakkeeran 2015). Major antimicrobial peptides produced by Bacillus sp. can be grouped into three categories, viz., iturins, surfactins, and fengycins/plipastatins. Iturin produced by B. subtilis, was effective against wide spectrum of fungal phytopathogens. A significant decrease in seed mycoflora was noticed in the seed loads that were tested with iturin A with concentrations of 50–100 ppm (Asaka and Shoda 1996). Treatment of corn seeds with iturin A @ 5 and 20 g/100 Kg showed a significant reduction in total microbial count was observed. B. subtilis strain RB14 that was capable of producing iturin B and surfactin that aided in suppressing damping-off disease of tomato (Asaka and Shoda 1996). In addition to disease control, B. subtilis strain BACT-O also promoted the plant growth and yield of cucumber (Utkhede et al. 1999). Liang et al. (2007) reported that seed priming with Bacillus polymixa increased the seedling height of safflower. Two types of chitinase enzymes are synthesized by B. amyloliquefaciens and can inhibit F. oxysporum growth (Wang et al. 2004). Chitinase enzymes with high chitinitic character are also produced by B. subtilis against various fungal pathogens (Chang et al. 2009). A chitinolytic bacterial strain (YC300), isolated from a compost sample from Republic of Korea, and produced an iturin-like compound. Later, this strain identified as Paenibacillus koreensis also provided a fair to good antifungal activities against Colletotrichum lagenarium, F. oxysporum and S. sclerotiorum, B. cinerea and R. solani (Chung et al. 2000). In an independent research, iturin A isolated from bacteria showed much stronger performance than surfactin against phytopathogens (Asaka and Shoda 1996). Three strains, B. cereus (L-07-01), B. subtilis (H-08-02), and Bacillus mycoides (S-07-01), exhibited significant antifungal activity against F. graminearum (Fernando et al. 2005; Ramarathnam et al. 2007). Soil application of Bacillus sp. was highly effective in the management of Fusarium wilt of carnation (Rajesh Kumar, 2014). B. subtilis, B. amyloliquefaciens, B. licheniformis, and B. cereus resulted in inhibiting the soil, air, and post-harvest plant diseases (Yoshida et al. 2002). Zwittermicin A is an aminopolyol compound produced by B. cereus and is also known to possess good inhibitory action against pathogenic fungi including oomycetes group of pathogens (Silo-Suh et al. 1998; Fernando et al. 2005). Delivering of talc-based consortia formulation comprising of B. subtilis (S2BC-2) + Burkholderia cepacia (TEPF-Sungal) reduced vascular wilt and corm rot of gladiolus under protected cultivation. Besides, increase in cormel and corm production and flowering was promoted upon the application (Shanmugam and Kanoujia, 2011). Kumar et al. (2014) reported the abiotic as well as biotic stress regulating potential of Bacillus sp. isolated from different rhizospheric zones of India. It was found that Bacillus sp. could control the growth of pathogenic fungal species such as Sclerotium rolfsii, Botrytis ricini, Macrophomina phaseolina, Fusarium oxysporum, and Rhizoctonia solani. On the other hand, this bacterium also demonstrated abiotic stress tolerance against salinity (NaCl 7%), higher temperature (50 °C), and drought (−1.2 MPa). Zwittermicin A is also popularly used as a broad spectrum of compound against different harmful microbes (Silo-suh et al. 1998). These groups of compounds also exhibit diverse biological activity against Oomycetous plant diseases as well as insecticidal activity (Emmert et al. 2004). Moreover, there are several reports which demonstrated the biological activity against different groups of plant pathogens by Bacillus species (Kloepper et al. 2004; Correa et al. 2009; Jogaiah et al. 2010). B. amyloliquefaciens with 23 diverse AMP genes effectively inhibited S. sclerotiorum which causes stem rot of carnation. Further, it significantly enhanced the plant growth and yield (Vinodkumar et al. 2015). In another study, the synergistic action of iturin and surfactin against Colletotrichum gloeosporioides was performed successfully (Kim et al. 2010). Surfactin like compounds isolated from B. subtilis (R14) and Bacillus circulans (Das et al. 2008; Fernandes et al. 2007) were found to suppress multidrug-resistant bacteria such as Escherichia coli, Alcaligenes faecalis, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, and methicillin-resistant. The four strains of B. subtilis which were effective against cucurbit powdery mildew also exhibited highest antibacterial activity against Xanthomonas campestris pv. cucurbitae and Pectobacterium carotovorum sub-sp. Carotovorum (Romero et al. 2007). These strains produced lipopeptide antibiotics, viz. fengycins, surfactins, and iturins. Further, thin-layer chromatography studies and direct bioautography revealed that the antibacterial activity was correlated due to iturin compound. This result was further validated using defective mutants of lipopeptide, thereby elucidated the importance of AMPs in plant disease control. US EPA (Environmental Protection Agency) has also provided a catalogue of biopesticides in which commercial formulations of distinct strains of Bacillus that can be employed as biocontrol agents are prescribed (McSpadden Gardener, 2004). These products are accessible in distinct types of preparations such as a wettable powder, dry cakes, or liquid or suspension in a liquid, as it depends on the nature of relationship between the biocontrol strain and carrier molecule. Products such as Taegro, Sublilex, Companion, Serenade, and Kodiak are all dependent on the utilization of various strains of B. subtilis as a biocontrol agent. Kodiak is known for the eradication of root inhabiting disease-causing agents of soybean and cotton like Aspergillus sp., Alternaria sp., Fusarium sp., and R. solani. Moreover, Serenade (Agra Quest, Daius CA, USA) constituting B. subtilis strain QST713 is known to remediate the Cercospora leaf spot, early and late blight diseases related with different crop plants. Similarly, bacterial strain, Bacillus sp. AZ-11 was also found as effective against reduction in Ganoderma growth. Several species of the genus Bacillus have been reported as potential BCAs. For instance, the bacterium B. tequilensis is reported to manage root knot nematode when co-inoculated with Trichoderma harzianum (Tiwari et al., 2017). Thus, this bacterium can have dual advantage in irrigated pockets where several vegetables suffer from root knot nematode. Incidentally, B. tequilensis is also known to solubilize Zinc for increased yield of wheat and soybean (Khande et al., 2017). In arid region, one strain of B. firmus has also been reported as specific antagonist to Macrophomina phaseolina (Lodha et al. 2013) in earlier studies. Bajoria et al. (2008) isolated B. subtilis, B. cereus, B. pumilus and B. sphaericus from hot arid region having biocontrol potential against plant pathogenic fungi. Therefore, the isolated antagonistic bacterial strains will be potentially very useful and yield effective results in further field studies for management of this terrific pathogen.
PLANT GROWTH PROMOTION
Bacillus sp. utilizes various means (direct as well as indirect) to promote plant growth. Bacillus sp. transforms the intricate or difficult form of indispensable nutrients, such as N and P, into very simple and bioavailable forms that can be taken up by the plant root system (Kuan et al. 2016; Shafi et al. 2017). The biological available form of Nitrogen in soils which is an essential constituent in nucleic acids, proteins, and other organic components, is partial or inadequate, which slows down plant growth in its natural ecosystem (Barker et al. 1974; De-Willigen 1986). There are some species of Bacillus sp. which release NH3 from nitrogenous organic matter (Hayat et al. 2010). Oliveira et al. (1993) reported that some of the Bacillus sp. possess the nif gene and demonstrate nitrogenase enzyme activity. This enzyme helps in the fixation of atmospheric Nitrogen and delivers it to the plants to augment plant development, growth, and yield by fulfilling N requirements and delaying the process of senescence (Seldin et al. 1984; Ding et al. 2005). The Bacillus sp. also excretes the proteinaceous compound known as a siderophore, which helps in iron chelation from the rhizospheric zone of plant (Wilson et al. 2006). The iron-chelating compound siderophores bind Fe+++ in complex substances and reduce the ferric form into ferrous form, which can easily enter plant root system (Dertz et al. 2006). Kumar et al. (2012) isolated seven soil bacterial isolates from bean rhizosphere in the Uttarakhand Himalayan region. These demonstrated excellent plant growth-promoting and biocontrol activities. On the bases of 16S rRNA gene sequence, the soil isolate was identified as Bacillus sp. and named BPR7. The rhizospheric strain BPR7 released phytohormone IAA, siderophores, enzyme phytase, organic acids, cyanogens, and ACC deaminase and was also able to solubilize numerous types of inorganic and organic phosphatic material as well as Zn and K. Gutiérrez-Mañero et al. (2008), reported that, B. pumilus and B. licheniformis isolated from alder (Alnus glutinosa [L.] Gaertn.) rhizosphere exhibited strong plant growth-promoting potential. Therefore, both the Bacillus bacteria demonstrated the good potential of promoting plant growth. The bioassay facts displayed that the dwarf phenotype produced in alder seedlings by a chemical paclobutrazol (which inhibits biosynthesis of gibberellin or GA) was reversed effectively by applying bacterial extracts from media and by exogenous treatment of GA3.
Calvo et al. (2010) isolated 63 Bacillus strains from the native potato rhizospheric regions growing in the Andean highlands of Peru. The strains demonstrated strong antagonism against Rhizoctonia solani and Fusarium solani. The antagonistic Bacillus strains were further verified for additional plant growth promotional aspects. It was observed that 81% of the isolates produced a certain amount of phytohormone indole-3-acetic acid (IAA) and 58% could solubilize tricalcium phosphate (TCP). The phylogenetic assessment revealed that the majority of isolates belonged to B. amyloliquefaciens sp., while other three potential strains may be novel putative Bacillus sp. Therefore, these Bacillus sp. may be used as potential potato growth promoters. Shakeel et al. (2015) obtained 234 rhizospheric isolates from basmati super rice and basmati-385 varieties cultivated in clay loam and saline soils from various locations of Punjab province in Pakistan. Out of the total 234 rice rhizospheric isolates, 27 isolates solubilized Zinc (Zn) from zinc oxide, zinc carbonate, and zinc phosphate. The soil isolate SH10 exhibited maximum zone of Zn solubilization (24 mm) using zinc phosphate, and soil isolate SH17 showed maximum zone of Zn solubilization (14–15 mm) using zinc oxide and zinc carbonate. Strains SH10 and SH17 also solubilized P (38–46 mm) and K (47–55 mm) under laboratory conditions. Both the strains also exhibited bio control activities against root rot (Fusarium moniliforme) and blast (Pyricularia oryzae) inciting plant pathogens by 22–29% and manufactured many biological control factors in vitro conditions. Bacterial strains SH10 and SH17 also enhanced Zinc uptake and translocation into the grains and augmented plant yield of super basmati rice and basmati-385 varieties by 18–47% and 22–49%, correspondingly. Using 16S rRNA gene analysis, both the SH10 and SH17 bacterial strains were identified as Bacillus sp. and Bacillus cereus. Idris et al. (2002) isolated many Bacillus strains belonging to B. subtilis or B. amyloliquefaciens from plant pathogen-contaminated soils and demonstrated PGPR properties. Soil isolate B. amyloliquefaciens could biodegrade extracellular compound phytate (myoinositol hexakisphosphate). The maximum phytase activity was found in isolate FZB45 (B. amyloliquefaciens), and the diluted bacterial cultural filtrate of this isolate encouraged maize seedling growth in phytate presence and P limitation. Lucas Garcia et al. (2004) studied the effect of inoculation of B. licheniformis on development and growth of tomato and pepper in three experiments. In the first experimental condition, the bacterium meaningfully enhanced plant height and the leaf surface area in both cultivars, and the effect was more on pepper as compared to tomato. In a second experiment, the plant seedlings were grown in sand, and hydroponic systems were tested. Interestingly, the size and number of tomato fruit increased after application of bacterial inoculation in sand and in the hydroponic system. Moreover, the inoculated cultivars exhibited less disease infestation as compared to untreated plants. In the third experiment, the pepper yield was greater in inoculated plants as compared to the uninoculated plants. This Bacillus strain had appreciable colonization and survival potential, and it could be employed as a biofertilizer as well as biocontrol agent. Kayasth et al. (2013) isolated Bacillus strain and designated as ML3 from a soil sample, and it produced the phytohormone and IAA and NH3 in the range of 174.72 and 0.66 μg ml−1, correspondingly. This strain also produced siderophores and effectively solubilized tricalcium phosphate (TCP). The main functional nitrogenase gene nif H was also found in this strain. Based on morphological, biochemical, and partial 16S rRNA gene sequencing, this strain ML3 was identified as B. licheniformis. This strain has all properties to be utilized as an efficient plant growth promoter. Agarwal and Agarwal (2013) isolated 28 bacterial strains from dissimilar tomato rhizospheric soils from Dehradun area of Uttarakhand, India. All of the soil isolates were characterized biochemically and tested for plant growth-promoting abilities such as indole acetic acid production (IAA), Phosphate (P) solubilization, HCN production, siderophore excretion, and catalase activity. Out of 28 soil isolates, only 5 Bacillus isolates exhibited potential plant growth-promoting abilities. Inoculation of tomato plants with selected Bacillus sp. resulted in increment in shoot and root length of tomato seedlings as compared to the uninoculated plants. Bacillus sp. also enhanced the seed germination percentage as well as seed vigor index. Ramírez and Kloepper (2010) have studied the consequence of inoculum concentration and soil phosphate-associated properties on plant growth encouragement by B. amyloliquefaciens strain FZB45, which produced phytase. A noteworthy synergy between soil phosphate and bacterial application was observed. B. amyloliquefaciens strain FZB45 encouraged plant growth and phosphate uptake, which confirms the role of enzyme phytase and less P uptake in uninoculated plants. This strain also produced IAA under laboratory conditions, but its role was not determined. Sharma et al. (2013) isolated a bacterium from the rhizosphere of soybean plants grown at Directorate of Soybean Research, Indore, Madhya Pradesh, India, and this bacterium was identified as Bacillus sp. on the basis of morphological and biochemical tests as well as FAME profile. Studies on 16S rRNA gene showed 98.7% homology to B. amyloliquefaciens, and thereafter, it was designated as strain sks-bnj-1 (AY 932823). This strain owned manifold plant growth-promoting attributes such as IAA production; siderophore production; ACC deaminase activity; enzymes like phosphatases, phytases, and cellulases; Zinc solubilization; and HCN production. This bacterium also exhibited biocontrol properties. Interaction of this strain with soybean increased the shoot root biomass as well as nutrient uptake as compared to the uninoculated control plants. In another study on Bacillus sp. by Cruz-Martín et al. (2015) conducted on banana plant in Cuba, it was observed that strain B. pumilus could fix atmospheric nitrogen and was able to grow in nitrogen-free culture media and produced IAA (28.9 μg ml−1). Moreover, strain B. pumilus significantly enhanced the plant height and thickness of the stem, altered root architecture, and improved fresh and dry plant weight. In the recent past, a novel bacterium, B. firmus was isolated from naturally heated cruciferous residue amended soil that showed antagonistic activity against M. phaseolina. In dual culture tests, it produced a scarlet pigmentation only against M. phaseolina (Lodha et al., 2013). Separate dual culture tests were also performed to ascertain the activity of antagonistic bacterium against prevalent soil fungi, Fusarium oxysporum f. sp. cumini and a bio-agent T. harzianum. However, B. firmus could not inhibit the growth of any of these fungi nor any scarlet pigmentation was observed during interaction. Bacillus sp. is particularly suited for studies on biological control due to its omnipresence in soils, high thermal tolerance, rapid growth in broth culture and ready formation of resistant spores. They are uniformly distributed throughout the soil rather than being concentrated in the plant rhizosphere. The main characteristics of B. firmus includes its thermal tolerance (45°C), phosphate solubilizing nature, compatibility with T. harzianum, increased nodulation, plant growth promotion and potential to colonize roots.
BIO FORMULATIONS FOR DISEASE SUPPRESSION
Many locally available on-farm wastes were evaluated to select food substrates that can improve the shelf life of B. firmus for a reasonable period. Our efforts successfully culminated in identifying a suitable food substrate, which was combined with carrier to retain adequate moisture in the bio-formulated product so that bacterium could survive for a period of 180 days. The product designated as Maru Sena 3 was made available for distribution to the farmers. The technology was validated after developing bio-formulated products and their efficacy was demonstrated at growers’ field to disseminate this eco-friendly and easy management strategy in the region (Mawar et al., 2018). Field demonstrations were carried out at adopted villages of the Institute’s Krishi Vigyan Kendra (Farm Science Centre dedicated for extension activities). Effectiveness of seed coating with bio-formulated product of B. firmus on incidence of dry root rot and seed yield of cluster bean, moth bean and sesame were demonstrated at grower’s field during last ten years. The percent increase in seed yield just by seed treatment ranged from 13.3-23.5% in all legumes and oil seed crops in different demonstrations. These products are made available for the farmers through Agricultural Technology Information Center (ATIC: Single window delivery mechanism) of the institute. In the last five years, hundreds of hectares have been sown with bacterium coated seeds. Farmers are getting positive response in checking incidence of dry root rot in rainfed crops of arid region namely cluster bean, cowpea, green gram, mothbean, sesame, etc. The process of developing bio-formulated product of these bio-pesticides has been patented in order to put these products for commercialization for wider adoption among growers. A patent “Bioformulation of a biopesticide and a process for preparing the same” was granted in 2019 with patent no. 309385.
Various studies reported that certain biocontrol consortia were unable to show at least comparable effects on plants when compared to their individual applications. One of the major causes for such contrary results may be attributed to incompatibility of the microbes in the mixture with each other. The findings clearly advocate for screening of compatible microbes for development of microbial consortia. The basic objective of developing microbial consortium would fail if the microbes used in the consortium do not have any additive or synergistic effects on disease suppression.
Application of bioagents in consortium may improve efficacy, reliability and consistency of the microbes under diverse soil and environmental conditions (Berg et al., 2005). Use of different species of biocontrol occupy different niches in the root zone and thereby restrict competition among them. Diversity in biocontrol mechanisms offered by each microbial component may also help in enhancing disease suppressiveness. A number of microbial consortia have been developed by many scientists and were tested in different crops in our country. Therefore, in the same stream our consortia prepared with two organisms worked in a synergistic manner. B. firmus operates through antibiosis while T. harzianum kills the mycelium of the pathogen mainly through hyper parasitism. It was, therefore, thought worthwhile to develop a consortium of native bio-control agents in the form of a single bio-formulated product to improve pathogen control by way of operating different mechanisms of antagonism for plant disease suppression. The biocontrol consortium activates the antioxidant enzyme activities and the phenylpropanoid pathway leading to accumulation of total phenolics, proline and pathogenesis related (PR) proteins after the pathogen challenge. The impact of triple microbial consortia consisting of fluorescent Pseudomonas (PHU094), Trichoderma (THU0816) and Rhizobium (RL091) for alleviation of biotic stress in chickpea is through enhanced antioxidant and phenylpropanoid activities (Akanksha et al., 2012). Periodical oxidase and accumulation of total phenol content was higher when challenged with the pathogen compared to the single microbe and dual microbial consortia. Pseuomonas aeruginosa PJHU15, Tricoderma harzianum TNHU27 and Bacillus subtilis BHHU100 as a consortium has been used to assess suppression of soft-rot pathogen Sclerotina sclerotiorum (Jain et al., 2012). The triple-microbe consortium and single-microbe treatments showed 1.4-2.3 and 1.1-1.7 fold increment in defense parameters respectively when compared to untreated challenged control. The compatible microbial consortia triggered defense responses in an enhanced level in pea than when the microbes were alone and provided better protection against Sclerotinia rot. Trichoderma species and fluorescent Pseudomonas sp. have been reported to induce systemic resistance in plants. These biological control agents were tested as a single application and in combination for their abilities to elicit induced resistance in cucumber against Fusarium oxysporum f. sp. radices cucumerinum and in A. thaliana against Botrytis cinerea. The combination of Tr6 and Ps14 induced a significantly higher level of resistance in cucumber, which was associated with the primed expression of a set of defense-related genes upon challenge with Fusarium. In Arabidopsis, both Ps14 and Tr6 triggered ISR against B. cinerea but their combination did not show enhanced effects. In the induced systemic resistance-defective Arabidopsis mutant myb72, none of the treatments protected against B. cinerea, whereas in the SA-impaired mutant sid2, all treatments were effective. Taken together, these results indicate that in Arabidopsis Ps14 and Tr6 activate the same signaling pathway and thus have no enhanced effect in combination. The enhanced protection in cucumber by the combination is most likely due to activation of different signaling pathways by the two biocontrol agents.
CONCLUSION AND PERSPECTIVES
Diversity in Bacillus species produce multiple classes of antimicrobial compounds inducing systemic resistance in a variety of ways that can be used for the plant growth promotion and management of a broad range of plant stresses and ultimately sustaining crop health. The spore forming ability of Bacillus species gives them a key importance in the field of biological control. The research areas that demand considerations for the successful adoption of Bacillus sp. as bioagents comprise the exploration for biodiverse antagonistic and antibiotic strains, elucidation of their mode of action and signalling pathways, stability under field application, development of effective formulations that can be used with other bioinoculants with synergetic effect and perfect demonstration of cost–benefit ratio for effective commercialization.
REFERENCES
Agrawal DP, Agrawal S (2013) Characterization of Bacillus sp. strains isolated from rhizosphere of tomato plants (Lycopersicon esculentum) for their use as potential plant growth promoting rhizobacteria. Int J Curr Microbiol Appl Sci 2(10):406–417
Ahmad M, Ahmad I, Hilger TH, Nadeem SM, Akhtar MF, Jamil M, Hussain A, Zahi ZA (2018) Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. Peer J 6:e5122.
Asaka O, Shoda M (1996) Biocontrol of Rhizoctonia solani damping off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol 62:4081–4085
Aseri GK, Jain N, Tarafdar JC (2009) Hydrolysis of organic phosphate forms by phosphatases and phytase producing fungi of arid and semi-arid soils of India. Am-Eurasian J Agric Environ Sci 5:564–570
Babalola OO, Glick BR (2012) Indigenous African agriculture and plant associated microbes: current practice and future transgenic prospects. Sci Res Essays 7:2431–2439
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19: 90–8
Bahadir PS, Liaqat F, Eltem R (2018) Plant growth promoting properties of phosphate solubilising Bacillus species isolated from the Aegean Region of Turkey. Turk J Bot 42:183–196
Bale JS, van Lenteren JC, Bigler F (2008) Biological control and sustainable food production. Philos Trans R Soc Lond B Biol Sci 363(1492):761–776
Barker AV, Maynard DN, Mills HA (1974) Variations in nitrate accumulation among spinach cultivars. J Am Soc Horticult Sci 99:32–134
Barr PO, Soila P (1960) Introduction of soft cannula into artery by direct percutaneous puncture. Observations on technique and cannula materials. Angiology 11:168–172
Calvo P, Ormeño-Orrillo E, Martínez-Romero E, Zúñiga D (2010) Characterization of Bacillus isolates of potato rhizosphere from Andean soils of Peru and their potential PGPR characteristics. Braz J Microbiol 41(4):899–906
Campbell R (1989) Biological control of microbial plant pathogens. Cambridge University Press, Cambridge, MA, pp 218
Chang WT, Chen ML, Wang SL (2009) An antifungal chitinase produced by Bacillus subtilis using chitin waste as a carbon source. World J Microbiol Biotechnol 26(5):945–950
Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim SD, Roberts DP (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soil borne pathogens of cucumber and pepper. Appl Microbiol Biotechnol 80:115–123
Chung YR, Kim CH, Hwang I, Chun J (2000) Paenibacillus koreensis sp. nov., a new species that produces an iturin-like antifungal compound. Int J Sys Evol Microbiol 50:1495–1500
Correa OS, Montecchina MS, Berti MF, Fernandez FMC, Pucheu NL, Kerber NL, Garcia AF (2009) Bacillus amyloliquefaciens BNM122 a potential microbial biocontrol agent applied on soybean seeds, cause a minor impact on rhizosphere and soil microbial communities. Appl Soil Ecol 41:185–194
Cruz-Martín M, Mena E, Sánchez-García C, Roque B, Acosta-Suárez M, Pichardo T, Leiva-Mora M, Alvarado-Capó Y (2015) The effects of plant growth promoting Bacillus pumilus CCIBPC5 on ‘Grande naine’ (Musa AAA) plants in acclimatization stage. Biotecnología Vegetal 15(3):151–156
Das P, Mukherjee S, Sen R (2008) Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J App Microbiol 104(6):1675–1684
Dertz EA, Stintzi A, Raymond KN (2006) Siderophore-mediated iron transport in Bacillus subtilis and Corynebacterium glutamicum. J Biol Inorg Chem 11(8):1087–1097
De-Willigen P (1986) Supply of soil nitrogen to the plant during the growing season. In: Lambers H, Neeteson JJ, Stulen I (eds) Fundamental, ecological and agricultural aspects of nitrogen metabolism in higher plants. Martinus Nijhoff, Dordrecht, pp 417–432
Ding Y, Wang J, Liu Y, Chen S (2005) Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J Appl Microbiol 99(5):1271–1281
Dipta B, Kirti S, Bhardwaj S, Gupta S, Kaushal R (2017) Phosphate solubilizing potential of Bacillus pumilus for the enhancement of cauliflower (Brassica oleracea var. botrytis l.). EM Int 23(3):1541–1548
Driouich A, Follet-Gueye ML, Vicr ´e-Gibouin M (2013) Root border cells and secretions as critical elements in plant host defense. Curr Opin Plant Biol 16:489–95
Earl AM, Losick R, Kolter R (2s008) Ecology and genomics of Bacillus subtilis. Trends Microbiol 16:269–275
Etesami H, Emami S, Alikhani H (2017) Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects-a review. J Soil Sci Plant Nutr 17
Farag MA, Ryu C-M, Sumner LW (2006) GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67:2262–8
Fernandes PAV, Arruda IRD, Santos AFABD, Araújo AAD, Maior AMS, Ximenes EA (2007) Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz J Microbiol 38(4):704–709
Fernando WGD, Ramarathnam R, Krishnamoorthy AS (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Fiddaman PJ, Rossall S (1995) Selection of bacterial antagonists for the biological control of Rhizoctonia solani in oilseed rape (Brassica napus). Plant Pathol 44:695–703
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Ann Rev Phytopathol 42:243–270
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica
Govindasamy V, Senthilkumar M, Magheshwaran V, Kumar U, Bose P, Sharma V, Annapurna K (2010) Bacillus and Paenibacillus sp.: potential PGPR for sustainable agriculture. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin/Heidelberg, pp 333–356
Grayston SJ, Wang S, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Gupta N, Sabat J, Parida R, Kerkatta D (2007) Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot Croat 66:197–204
Gutiérrez‐Mañero FJ, Ramos B, Probanza A, Mehouachi J, Talon M (2008) The plant growth promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberelins. Physiol Plant 111:206–211
Han SH, Lee SJ, Moon JH (2006) GacS-dependent production of 2R, 3R-Butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant Microbe In 19:924–30
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Idris EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Richter T, Borriss R (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth- promoting effect. Microbiology 148:2097–2109
Idris EE, Bochow H, Ross H, Borriss R (2004) Use of Bacillus subtilis as biocontrol agent. VI. Phytohormone action of culture filtrate prepared from plant growth promoting Bacillus amyloliquefaciens FZB24, FZB42, FZB45 and Bacillus subtilis FZB37. J Plant Dis Prot 111:583–597
Idris EE, Iglesias DJ, Talon M (2007a) Tryptophan dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant-Microbe Interact 20(6):619–626
Idris EES, Iglesias DJ, Talon M, Borriss R (2007b) Tryptophan-dependent production of Indole-3- Acetic Acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact 20:619–626
Jadhav RN (2016) Study of phosphate solubilization and antimicrobial activity of Bacillus licheniformis isolated from rhizosphere of Cajanus cajan cultivated in Marathwada region. Life Sci Int Res J 3(2):129–132
Jogaiah S, Roopa KS, Pushpalatha HG, Shekar Shetty H (2010) Evaluation of plant growth-promoting rhizobacteria for their efficiency to promote growth and induce systemic resistance in pearl millet against downy mildew disease. Arch Phytopathol Plant Prot. 43:368– 378
Johri BN, Sharma A, Virdi JS (2003) Rhizobacterial diversity in India and its influence on soil and plant health. Adv Biochem Eng Biotechnol 84:49–89
Kai M, Haustein M, Molina F (2009) Bacterial volatiles and their action potential. Appl Microbiol Biot 81:1001–12
Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH (2014) Phosphate solubilising Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol 54(4):427–433
Kayasth M, Kumar V, Gera R (2013) Exploring the potential of PGPR strain Bacillus licheniformis to be developed as multifunctional biofertilizer. Cent Eur J Exp Biol 2(1):12–17
Khan MS, Ahmad E, Zaidi A, Oves M (2013) Functional aspect of phosphate-solubilizing bacteria: importance in crop production. In: Maheshwari DK (ed) Bacteria in agrobiology: crop productivity. Springer, Berlin, pp 237–265
Kibrom FG, Alemayehu W, Prakasam VR, Kiros W (2017) Isolation and characterization of efficient phosphate solubilizing Bacillus (PSB) from different agro-ecological zones of Tigray Soil, Ethiopia. Momona Ethiop J Sci 9(2):262–273
Kim PI, Ryu J, Kim YH, Chi YT (2010) Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20(1):138–145
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus sp. Phytopathology 94:1259–1266
Kuan KB, Othman R, Rahim KA, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 11:e0152478
Kumar BSD (1999) Fusarial wilt suppression and crop improvement through two rhizobacterial strains in chickpea growing in soils infested with Fusarium oxysporum f.sp. ciceris. Biol Fertil Soils 29:87–91
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167(8):493–499
Lee B, Farag MA, Park HB (2012) Induced resistance by a longchain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS One 7:e48744
Liang BH, Shi ZQ, Zhang T, Yang ZM (2007) Fengycin antibiotics isolated from Bacillus sp. FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol Lett 272:91–98
Lodha S, Mawar,R. Chakarbarty P K and Bhagvan Singh (2013). Managing Macrophomina phaseolina causing dry root rot of legumes by a native strain of Bacillus firmus. Indian phytopathology 66 (4) : 356-360
Lucas Garcia JA, Probanza A, Ramos B, Ruiz Palomino M, Gutiérrez Mañero FJ (2004) Effect of inoculation of Bacillus licheniformis on tomato and pepper. Agronomie 24:169–176
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63: 541–56
Maheswar NU, Sathiyavani G (2012) Solubilization of phosphate by Bacillus Sps, from groundnut rhizosphere (Arachis hypogaea L). J Chem Pharm Res 4(8):4007–4011
Maougal RT, Brauman A, Plassard C, Abadie J, Djekoun A, Drevon JJ (2014) Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. Eur J Soil Biol 62:8–14
Matos ADM, Gomes ICP, Nietsche S, Xavier AA, Gomes WS, Neto JADS, Pereira MCT (2017) Phosphate solubilization by endophytic bacteria isolated from banana trees. Anais da Academia Brasileira de Ciências 89(4):2945–2954
McInroy JA, Kloepper JW (1995) Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 173:337–342
McKenzie RH, Roberts TL (1990) Soil and fertilizer phosphorus update. In: Alberta soil science workshop proceedings of coast terrace inn Edmonton, AB 84 104 20–22 Feb
Mcspadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus species in agricultural systems. Phytopathology 94:1252–1258
Mihailovi V, Vukovi N, Nićiforovi N, Soluji S, Mladenovi M, Mascaron P, Stankovi MS (2011) Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L. J Med Plants Res 5(7):1164–1174
Mishagi IJ, Donndelinger CR (1990) Endophytic bacteria in symptom free cotton plants. Phytopathology 9:808–811
Mohamed EAH, Farag AG, Youssef SA (2018) Phosphate solubilization by Bacillus subtilis and Serratia marcescens isolated from tomato plant rhizosphere. J Environ Prot 9(3):266–277
Montesinos E, Bonaterra A (2009) Microbial pesticides. In: Schaechter M (ed) Encyclopedia of microbiology, 3rd edn. Elsevier, pp 110–120
Mora I, Cabrefiga J, Montesinos E (2011) Antimicrobial peptide genes in Bacillus strains from plant environments. Int Microbiol 14:213–223
Musthafa KS, Balamurugan K, Pandian SK, Ravi AV (2012) 2,5-Piperazinedione inhibits quorum sensing-dependent factor production in Pseudomonas aeruginosa PAO1. J Basic Microbiol 52 (6):679–686
Nakkeeran S, Kavitha K, Mathiyazhagan S, Fernando WGD, Chandrasekar G, Renukadevi P (2004) Induced systemic resistance and plant growth promotion by Pseudomonas chlororaphis strain PA-23 and Bacillus subtilis strain CBE4 against rhizome rot of turmeric (Curcuma longa L.). Can J Plant Pathol 26:417–418
Nakkeeran S, Renukadevi P, Marimuthu T (2005) Antagonistic potentiality of Trichoderma viride and assessment of its efficacy for the management of cotton root rot. Arch Phytopathol Plant Prot 38(3): 209–225
Oliveira SS, Seldin L, Bastos MC (1993) Identification of structural nitrogen-fixation (nif) genes in Bacillus polymyxa and Bacillus macerans. World J Microbiol Biotechnol 9(3):387–389
Ongena M, Jacques P (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–25
Ortiz-Castro R, Valencia-Cantero E, Lopez-Bucio J (2008) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3:263–5
Pal KK, Gardener BM (2006) Biological control of plant pathogens. Plant Health Instruct. 2:1117–1142
Perez-Garcia A, Romero D, de Vincente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotech 22: 187–93
Pirttijärvi TS, Andersson MA, Salkinoja-Salonen MS (2000) Properties of Bacillus cereus and other bacilli contaminating biomaterial-based industrial processes. Int J Food Microbiol 60(2–3):231–239
Pleban S, Chernin L, Chet I (1997) Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett Appl Microbiol 25:284–288
Rajesh Kumar P (2014) Role of antimicrobial peptide genes of PGPR and fungicides for the management of stem rot of and wilt complex of carnation under protected cultivation. M.Sc. thesis, Tamil Nadu Agricultural University, Coimbatore, India
Rajesh Kumar P, Adhipathi P, Nakkeeran S (2014) Antimicrobial peptide genes of PGPR for the management of Fusarium wilt of carnation protected cultivation. 44:54–58
Ramani V, Patel HH (2011) Phosphate solubilization by Bacillus sphaericus and Burkholderia cepacia in presence of pesticides. J Agric Technol 7(5):1331–1337
Ramarathnam R, Bo S, Chen Y, Fernando WGD, Xuenwen G, De Kievit T (2007) Molecular and biochemical detection of fengycin and bacillomycin D producing Bacillus sp. antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53:901–911
Ramírez CA, Kloepper JW (2010) Plant growth promotion by Bacillus amyloliquefaciens FZB45 depends on inoculum rate and P-related soil properties. Biol Fertil Soil 46(8): 835–844
Reva O, Smirnov VV, Petterson B, Priest FG (2002) Bacillus endophyticus sp. nov., isolated from the inner tissues of cotton plants (Gossypium sp.). Int J Syst Evol Microbiol 52:101–107
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Romero D, Vicente A, Rakotoaly RH, Dufour SE, Veening JW, Arrebola E, Cazorla FM, Kuipers O, Paquot M, Garcia AP (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis towards Podosphaera fusca. Mol Plant Microbe Interact 118(2):323–327
Ryan P, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Phys 52:527–60
Ryu CM, Farag MA, Hu CH (2003) Bacterial volatiles promote growth in Arabidopsis. P Natl Acad Sci USA 100:4927–32
Ryu CM, Farag MA, Hu CH (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134: 1017–26
Sadashiva CT, Sharanappa P, Rema shree B, Raghu AV, Udayan PS, Balachandran I (2010) Chemical composition and antimicrobial activity of the essential oil from bark of Pittosporum dasycaulon Miq. Adv Biol Res 4:301–304
Sathyaprabha G, Kumaravel S, Ruffina D, Praveenkumar P (2010) A comparative study on antioxidant, proximate analysis, antimicrobial activity and phytochemical analysis of Aloe vera and Cissus quadrangularis by GC-MS. J Pharm Res 3(12):2970–2973
Seldin L, Van Elsas JD, Penido EGC (1984) Bacillus azotofixans sp. nov. a nitrogen-fixing species from Brazilian soils and grass roots. Int J Syst Bacteriol 34(4):451–456
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnological Equip. https://doi.org/10.1080/13102818.2017.1286950
Shakeel M, Rais A, Hassan MN, Hafeez FY (2015) Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati Rice (Oryza sativa) varieties. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01286
Sharga BM (1997) Bacillus isolates as potential biocontrol agents against chocolate spot on faba beans. Can J Microbiol 43:915–924
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer plus 2:587
Sharma SK, Ramesh A, Johri BN (2013) Isolation and characterization of plant growth-promoting Bacillus amyloliquefaciens strain sks_bnj_1 and its influence on rhizosphere soil properties and nutrition of Soybean (Glycine max L. Merrill). J Virol Microbiol 2013:446006. https://doi. org/10.5171/2013.446006
Shishido M, Breuil C, Chanway CP (1999) Endophytic colonization of spruce by plant growth promoting rhizobacteria. FEMS Microbiol Ecol 29:191–196
Silo-suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J (1994) Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW 85. Appl Environ Microbiol 60:2023–2030
Silo-suh LA, Stab VE, Raffel SR, Handelsman J (1998) Target range of Zwittermicin A, an Aminopolyol antibiotic from Bacillus cereus. Curr Microbiol 37:6–11
Sparks DL, Huang PM (1985) Physical chemistry of soil potassium. In: Potassium in agriculture, pp 201–276
Swain MR, Laxminarayana K, Ray RC (2012) Phosphorus solubilization by thermotolerant Bacillus subtilis isolated from cow dung microflora. Agric Res 1(3):273–279
Tahir M, Mirza MS, Zaheer A, Dimitrov MR, Smidt H, Hameed S (2013) Isolation and identification of phosphate solubilizer Azospirillum, Bacillus and Enterobacter strains by 16SrRNA sequence analysis and their effect on growth of wheat (Triticum aestivum L.). Aust J Crop Sci 7(9):1284–1292
Tallapragada P, Seshachala U (2012) Phosphate-solubilizing microbes and their occurrence in the rhizospheres of Piper betel in Karnataka. India Turk J Biol 36:25–35
Tarafdar JC, Rao AV, Bala K (1988) Production of phosphatases by fungi isolated from desert soils. Folia Microbiol 33:453–457
Turan M, Ataoğlu N, Şahin F (2007) Effects of Bacillus FS-3 on growth of tomato (Lycopersicon esculentum L.) plants and availability of phosphorus in soil. Plant Soil Environ 53(2):58–64
Utkhede RS, Koch CA, Menzies JG (1999) Rhizobacterial growth and yield promotion of cucumber plants inoculated with Pythium aphanidermatum. Can J Plant Path 21:265–271
Van Loon L, Bakker P, Pieterse C (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–83
Vinodkumar S, Nakkeeran S (2015) Synergistic action of antimicrobial peptide (AMP) genes in Bacillus amyloliquefaciens for the management of stem rot of carnation. J Mycol Plant Pathol 45(4):229–234
VinodKumar S, Rajesh Kumar P, Senthilraja C, Nakkeeran S, Fernando WGD (2015) First report of Sclerotinia sclerotiorum causing stem rot of carnation (Dianthus caryophyllus) in India. Plant Dis 99(9):1280
Walker TS, Bais HP, Grotewold E (2003) Root exudation and rhizosphere biology. Plant Physiol 132: 44–51
Wang J, Liu J, Wang X (2004) Application of electrospray ionization mass spectrometry in rapid typing of fengycin homologues produced by Bacillus subtilis. Lett Appl Microbiol 39 (1): 98–102
Wang T, Liu MQ, Li HX (2014) Inoculation of phosphate solubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agric Scand B Soil Plant Sci 64(3):252–259
Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506
Wilson MK, Abergel RJ, Raymond KN, Arceneaux JE, Byers BR (2006) Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem Biophys Res Commun 348(1):320–325
Xie X, Zhang H, Pare PW (2009) Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03). Plant Signal Behav 4: 948–53
Yadav RS, Tarafdar JC (2003) Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol Biochem 35:1–7
Yoshida S, Shirata A, Hiradate S (2002) Ecological characteristics and biological control of mulberry anthracnose. Jpn Agric Res Q 36:89–95
Zhang H, Kim MS, Krishnamachari V (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–51
Berg G, Eberl L and Hartmann A. (2005) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7, 1673–1685.
Akanksha S, Sarma BK, Upadhyay RS and Singh HB. (2012). Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiological Research 168, 33-40.
Jain A, Singh S, Sarma B Kumar and Singh HB. (2012). Microbial consortium–mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. Journal of Applied Microbiology 112(3):537-50.
Mawar R, Tomer A S and Dheeraj Singh (2018). Demonstration of bio-formulated products of bio agents against disease incidence and seed yield of crops in Indian arid region. Indian Phytopathology 71:1-6
Mawar R, and Lodha S. 2017. Native Bio-agents: An integral component for managing soil borne plant pathogens in arid region. In: Microbial Antagonists: their role in biological control of plant diseases. Today &Tomorrow’s Printers and Publishers, New Delhi-110 002, India
Mawar, R., Ram, L., Sharma, D. and Jangid, K., 2021. Bacterial antagonists against Ganoderma lucidum the incitant of root rot of Indian Mesquite.Indian Phytopathology, pp.1-6.