DOI: https://doi.org/10.56669/LRKM6748
ABSTRACT
Biological control offers a promising and safer approach to protect plants from pathogens. Bio-control agents provide effective control of diseases by adopting various strategies like competition, antibiosis, hyperparasitism etc. But they exhibit inconsistent performance under diverse field conditions, which demands for the search of novel approaches in biological control of plant diseases. Under such circumstances, microbial consortia which exhibit diverse modes of action and have synergistic effect in enhancing efficacy of bio-control agents can be an effective strategy in combating plant diseases. Microbial consortia in addition to disease suppression, contributes to plant growth promotion, nutrient assimilation and bioremediation. Despite of being versatile in action, microbial consortia face several challenges with respect to incompatibility, regulatory issues which have to be addressed for their effective implementation in managing diseases. This review focuses on potentiality of microbial consortia over single inoculants in managing diseases, microbial interactions within consortia, mechanism of action, challenges in formulation and commercialization of microbial consortia.
Keywords: Biological control, Diseases, Efficacy, Microbial consortia.
INTRODUCTION
Global food demand is increasing rapidly, whereas agricultural production is facing many uncertainties such as climate change, abiotic stresses and biotic stresses such as insects, diseases and nematodes. Plant diseases pose serious threat to global food production, as evidenced by historical pandemics such as Irish famine and Great Bengal famine (Ristaino et al., 2021). Chemical pesticides have been used extensively to manage diseases, but chemical pesticides pose environmental hazards and adversely affects non-target organisms (Khan et al., 2023). Owing to the consumers demand towards low or zero chemical residues and regulatory restrictions on permitted level of chemical residues, biological control agents (BCA) based control of plant diseases are emerging as effective tools for the management of diseases. Different mechanisms of action exhibited by bio-control agents such as competition, antibiosis, mycoparasitism and induction of plant defence play crucial role in sustainable management of diseases (Palmieri et al., 2022). However, single strain inoculation of bio-control agents was not found to be effective under diverse field conditions. The Potentials of bio-control agents can be enhanced using mixtures of different bio-control agents exhibiting synergistic effect (Poveda et al., 2022). Application of microbial consortia improves efficacy and consistency of microbes under diverse environmental conditions. Varied mechanisms of action exhibited by each of bio-control agents in consortia enhances disease suppressiveness of microbial consortia (Kumar and Jagadeesh, 2016). Thus, microbial consortia can be a promising approach for sustainable management of diseases.
MICROBIAL CONSORTIA
Microbial consortia are a diverse group of microbes that co-exist and interact with each other (Lashani et al., 2023). Microbial consortia have diverse roles in agriculture like bioremediation of pesticides (Jariyal et al., 2018), plant biomass degradation (Cortes-Tolalpa et al., 2018), plant growth promotion (Santoyo et al., 2021) and disease suppression (BCA consortia). BCA consortia comprise of different bacterial antagonist or fungal antagonist or both. Consortia comprising of bacterial antagonist Pseudomonas fluorescens (NAIMCC-B-01981), fungal antagonist Trichoderma harzianum (MH027645.1) and endophyte Neofusicoccum parvum exhibited maximum inhibition of Sclerotium rolfsii compared to single antagonist (Jadav et al., 2022). In contrary to a single strain, BCA consortia are consistent under diverse crop conditions. Thus, efficacy of bio-control agents under field conditions can be improved by the usage of a mixture of BCAs, and synergistic effect of different BCAs can be exploited (Hegde and Vijaykumar, 2022).
Microbial consortia have several advantages in plant disease management over the use of a single strain such as i) Consortia of bio-control agents targets wide range of phytopathogens (Thakkar and Saraf, 2015), ii) Improves soil suppressiveness by increasing microbial diversity in soil rhizosphere (Palmieri et al., 2017), iii) Diverse species occupy different ecological niches in rhizosphere and there by restricts competition between them (Thomloudi et al., 2019), iv) Microbe interactions in consortium enhances rhizosphere colonization (Wu et al., 2023), v) Employing multiple strains of microorganisms with diverse modes of action improves bio-control efficiency of consortia (Thakkar and Saraf, 2015).
MICROBIAL INTERACTIONS AND THEIR EFFECT ON DISEASE MANAGEMENT
Microorganisms are ubiquitous and have diverse roles in agriculture by interacting with each other in rhizosphere region and with plants as well in phyllosphere region and contribute to biotic and abiotic stress management. Through their interactions, they can efficiently colonize rhizosphere and certain beneficial plant microbiota can even enhance disease resistance. Thus, microbial interactions have direct as well as indirect influence on the suppression of plant diseases.
Elevated production of antimicrobial substances
Bio-control agents have direct antagonistic effect on pathogens by production of toxic antimicrobial substances like bacteriocins, lipopeptides (iturin, fengycin and surfactin), chitinase and killer toxins. Single strain may produce low concentration of antimicrobial substance, whereas microbe interactions can boost its production to higher level (Brakhage, 2013). 2,4-diacetylphloroglucinol (DAPG) from P. fluorescens strain CHA0 enhanced production of N-acetyl-β-d-glucosaminidase in Trichoderma P1 strain when co-cultured (Lutz et al., 2004). Rhizobacterial isolates comprising of Stenotrophomonas, Serratia, and Bacillus species can produce complex blend of VOC’s and inhibits growth of phytopathogenic fungi (Vespermann et al., 2007). Microbial interactions are also known to produce novel antimicrobial compounds in addition to elevating their production (Niu et al., 2020). A novel antibiotic amycomicin has been identified to be produced by interaction between Amycolatopsis sp. AA4 and Streptomyces coelicolor M145 (Pishchany et al., 2018).
Efficient rhizosphere colonization
Efficient colonization of rhizosphere by bio-control agents acts as first line of defence against soil borne pathogens. Mixture of four bacterial isolates comprising of Serratia marcescens, Pseudomonas fluorescens, Rahnella aquatilis and Bacillus amyloliquefaciens improved soil suppressiveness against Fusarium oxysporum f. sp. ciceris race 0 and Fusarium solani f. sp. pisi causing Fusarium decline in chickpea by increasing microbial diversity in rhizosphere (Palmieri et al., 2017). Garbeva et al. (2014) reported volatile mediated interaction between different soil bacteria as Collimonas pratensis, Serratia plymuthica, Paenibacillus spp. and Pedobacter spp. stimulated the growth of Pseudomonas fluorescens and further C. pratensis triggered, antimicrobial secondary metabolite production in P. fluorescens. Thus, microbial consortia can improve soil health by increasing microbial diversity in rhizosphere.
Synergistic biofilm formation
Biofilm is a community of microorganisms in which cells adhere to each other or also to a surface which are embedded in extracellular matrix. Bio-film formation takes place through cell-to-cell communication in microbes by quorum sensing. Bio-film formation potentially occupies sites that can be invaded by pathogen and thus causes spatial and nutritive competition to pathogens. Chen et al., (2024) reported that altered molecular conformations and increased exoproteins, exopolysaccharides in microalgal-bacterial consortium enhanced biofilm formation. Streptomyces pactum improved bio-film formation, enhanced nutrient utilization, environmental adaptation in Pseudomonas koreensis and boosted its competitiveness (Guo et al., 2020).
Motility
Dispersal of fungal spores on bacteria and bacterial dispersal takes place through fungal mycelium in rhizosphere. The mycelia of Trichoderma can serve as a supporting layer for formation of bacterial biofilms and can aid bacterial migration in the soil (Niu et al., 2020). Motility helps BCA to colonize rhizosphere region and supress soil borne pathogens.
Microbial interactions through their synergistic effect can thus improve efficacy of bio-control agents. Diverse group of microorganisms can deplete resources rapidly than single strain alone and causes competitive inhibition of pathogens. In addition, microbes exhibit syntrophic interaction (cross feeding), wherein both microbes together carry out metabolism of single complex substrate which neither one can do alone. Thus, syntrophic interaction can enhance survival of BCA by efficient utilization of nutrients in its vicinity.
MODE OF ACTION
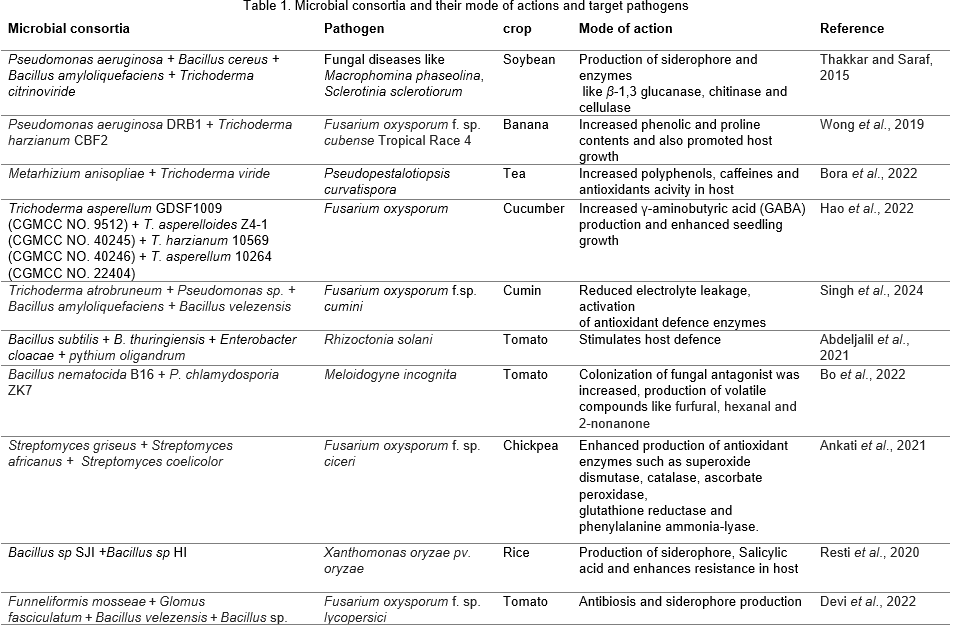
Bio-control agents have direct as well as indirect antagonistic mechanisms against pathogens like hyperparasitism, antibiosis, competition for space and nutrients, production of volatile organic compounds, toxin production and inducing systemic resistance in plants. BCA consortia may exhibit diverse mode of action and are consistent in action compared to single strain of BCA. Mode of action of certain microbial consortia are shown in Table 1.
MICROBIAL CONSORTIA AND THEIR ROLE IN ENHANCING PLANT RESISTANCE
Plants for their better acclimatization for biotic and abiotic stresses have evolved into various mechanisms. When attacked by pathogens, plants activate their defence responses rapidly to protect themselves. Induction of host resistance is indirect mechanism of action exhibited by BCA. Exogenous application of BCA elicits defence responses in plants like production of phenolic compounds, antioxidant enzymes, pathogenesis related enzymes and induction of systemic resistance. Whereas, priming with several beneficial microorganisms can elevate extent and duration of activation of defence responses.
Production of antioxidant enzymes
Plants in response to attack by pathogens as an immediate response, leads to rapid accumulation of reactive oxygen species (ROS). When produced in controlled manner they are involved in signal transduction but abruptive production of ROS can cause disruption of normal metabolism and key cellular functions. BCA can regulate ROS levels by production of antioxidant enzymes like superoxide dismutase (SOD) and peroxidase (POD) and provides protection against diverse plant pathogens (Xu et al., 2008). Ram et al. (2019) showed that consortium comprising of Trichoderma harzianum TNHU27 and Pseudomonas aeruginosa PJHU15 were effective in regulating antioxidant activity in cauliflower against Sclerotinia sclerotiorum causing collar rot in cauliflower and was superior to single BCA inoculation by induction of enhanced and faster defence response. Singh et al. (2013) stated rhizospheric microbial consortium consisting of fluorescent Pseudomonas (PHU094), Trichoderma (THU0816) and Rhizobium (RL091) strain increased phenylalanine ammonia lyase, polyphenol oxidase, accumulation of total phenol content in chickpea against Sclerotium rolfsii causing collar rot in triple microbial consortium as compared to dual microbial consortium and single microbe inoculation.
Activation of systemic resistance
Plants in response to attack by pathogens shows hypersensitive response which further induces systemic acquired resistance (SAR) mediated by salicyclic acid signalling leading to accumulation of PR proteins having antimicrobial activities. On the other hand, beneficial rhizobacteria triggers induced systemic resistance (ISR) mediated by Jasmonic acid (JA) and ethylene. Both SAR and ISR are effective against wide range of pathogens.
Bacillus amyloliquefaciens CECT 8238 and Rhizophagus irregularis consortia were able to induce ISR against Botrytis cinerea in tomato by reducing the area of the necrotic lesions by 33-37% (Minchev et al., 2021). Seed treatment with consortia comprising of 1% Trichoderma harzianum + 2% Pseudomonas fluorescens + 2% Bacillus subtilis induced 1.4%, 1.6%, 2.3% increase in peroxidase, polyphenol oxidase and total phenol in chickpea respectively and showed elevated production of defence related enzymes as compared to single BCA application (Trivedi et al., 2020). Activation of defence response was observed in potato plants for protection against early blight caused by Alternaria solani by increased accumulation of pathogenesis related proteins like chitinase and β-1,3-glucanase in triple microbial consortia compared to other treatments (Kumar et al., 2023).
Production of Phenolic compounds
Phenolic compounds are group of metabolites that can alter various physiological process in plants. Phenols participate in lignin deposition and act as mechanical barrier for penetration of pathogens. Bacillus subtilis BHHU100, Pseudomonas aeruginosa PJHU15 and Trichoderma harzianum TNHU27 increased accumulation of phenolics like chlorogenic acid, p-coumaric acid, syringic acid, cinnamic acid, gallic acid, shikimic acid in collar and leaf region and enhanced protection against Sclerotinia sclerotiorum in pea (Jain et al., 2015). Two strains of Trichoderma viz., phyllosphere isolate BHUF4 and rhizospheric isolate T16A were effective in protecting chilli plants from infection of Colletotrichum truncatum by accumulation of phenolics like kaempeferol (6.22-fold), capsaicin (16.1-fold), quercetin (5.36-fold) and salicylic acid (94.88-fold) (Saxena et al., 2020).
MICROBIAL CONSORTIA IN DETOXIFICATION OF MYCOTOXINS
Mycotoxins are toxic metabolites produced by certain molds or fungi that can contaminate food and feed and pose significant health risks to humans and animals, ranging from acute poisoning to chronic diseases. Some of the common mycotoxins produced in grains and nuts are aflatoxins, ochratoxins, fumonisins, zearalenone (ZEN) and deoxynivalenol (DON). Significant portion of postharvest losses is due to fungal diseases and mycotoxin production. The role of mycotoxins in the progression of diseases suggests that reduction of mycotoxins can be a valuable approach for management of diseases. Use of fungicides is not desirable as it can have residual effect and hence use of microbes for detoxification is a promising approach. (Sanzani et al., 2016).
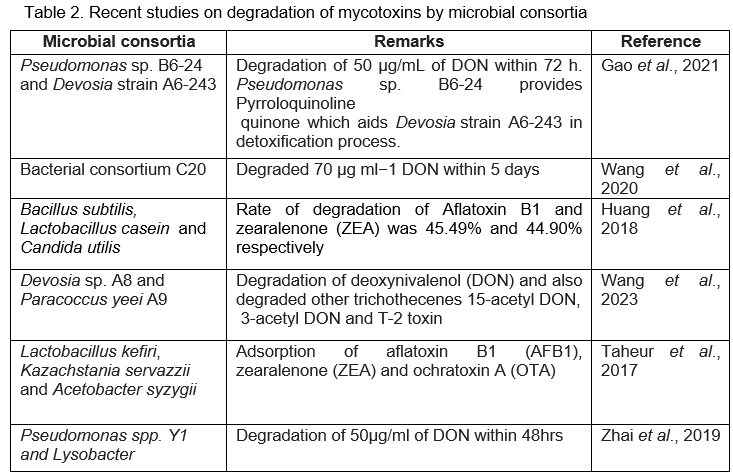
Synthetic microbial consortia exhibit greater efficiency in detoxifying mycotoxins compared to monocultures. Synergistic interactions among various species within microbial consortia promotes specific catalytic reactions leading to efficient mycotoxin detoxification. Concurrent transformation of diverse mycotoxins remains difficult for individual strains because of the chemical diversity of mycotoxins. However, using a combination of different microbes, each capable of performing distinct functions, offers a better strategy to reduce the risk of contamination by multiple mycotoxins. The effectiveness of surface adsorption of mycotoxins can be significantly improved through microbial consortia (Wang et al., 2023). In recent days, substantial research has focused on the detoxification of mycotoxins using microbial consortia and some of them are detailed in Table 2.
FORMULATIONS AND APPLICATION METHODS
Different application methods of microbial consortia such as seed treatment, foliar application and soil application are proved to be effective in managing diseases. Despite of having high potentiality in suppressing disease, formulations and application methods plays crucial role in determining their efficacy. Composts when amended with Bacillus subtillis B5, Anabaena oscillarioides C12 and compost tea preparations were effective in reducing severity of root diseases of tomato caused by Rhizoctonia solani, Pythium aphanidermatum, Fusarium oxysporum and Pythium debaryanum (Dukare et al., 2011). Kumar et al. (2015) developed seed coating formulations of B. subtilis OTPB1 + T. harzianum (OTPB3) using vermicompost has carrier material and found that microbial consortia formulation upregulated defence related enzymes such as superoxide dismutase, peroxidase and polyphenol oxidase and reduced lesion size caused by Alternaria solani and Phytophthora infestans in tomato. Consortia comprising of B. subtilis (Km1), P. fluorescens Pf1 and T. viride (TNAU) through soil application using talc as carrier material along with farmyard manure was proved to be effective in managing leaf blight of coconut caused by Lasiodiplodia theobromae (Johnson et al., 2017). Maheshwari et al. (2015) proved that vermiculite based consortial bioformulation consisting of Bacillus licheniformis KRB1 and Pseudomonas aeruginosa KRP1 had higher viability of bacteria and was antagonistic against Fusarium oxysporum and Sclerotinia sclerotiorum. Pesta granules application of consortium of Pseudomonas aeruginosa DRB1 and Trichoderma harzianum CBF2 reduced disease severity of fusarium wilt of banana by 66.67% (Wong et al., 2019). Drenching of Trichoderma viride + Bacillus subtilis + Pseudomonas flourescens consortia along with neem cake and molasses was highly effective in controlling bacterial wilt of tomato caused by Ralstonia solanacearum and disease incidence was not observed in treated plot (Sood et al., 2021). Liquid organic fertilizers formulated using cattle manure, rice straw, vegetable and fruit waste enriched with consortium of Trichoderma sp., Pseudomonas putida Pf10 and Bacillus subtilis JB12 when applied through combination of all three application methods viz., seed treatment, seedling dip and foliar spray reduced disease severity of bacterial leaf blight by 21% and blast of rice by 4% (Nurcahyanti et al., 2024).
MICROBIAL CONSORTIA FOR SUSTAINABLE AGRICULTURAL PRODUCTION
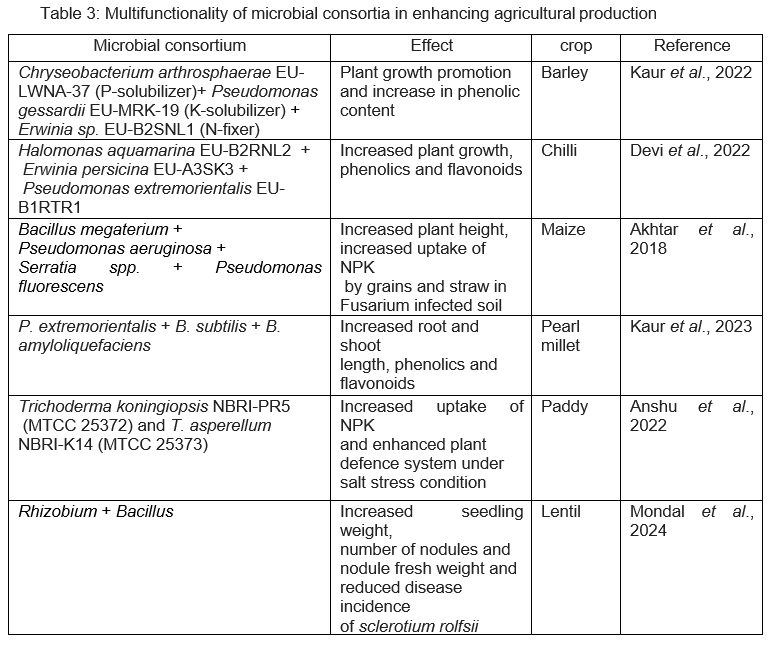
Plant growth promoting rhizobacteria (PGPRs) and their interaction with plants have tremendous role in sustainable agricultural production. They have diverse roles like plant growth promotion, mitigation of abiotic stresses like salt and drought tolerance, siderophore production and nutrient solubilisation. Plant growth promotion is attributed to their ability to produce gibberellins, cytokinins, auxins and ethylene. Ethylene apart from stimulating plant growth, is involved in defence signalling and inducing resistance in plants. Nutrient assimilation is important trait exhibited by microbes like N2 fixation by Rhizobia species through symbiotic relationship with plants and siderophore production which makes iron available to plants (Vishwakarma et al., 2020). Some of the multifunctionality traits of microbial consortia are presented in Table 3.
COMMERCIAL FORMULATIONS OF MICROBIAL CONSORTIA
Owing to many advantages of microbial BCA consortia like consistency, longevity, modularity, acclimatization to diverse environmental conditions and its capacity to target a wide range of pathogens, formulations comprising of multiple BCA are gaining importance. Bio-control product based on microbial consortia (BIO-TAM 2.0) comprising of Trichoderma asperellum and T. gamsii was first registered in 2015 and was known to be effective against Verticillium spp., Phytophthora spp., Pythium spp., Sclerotium rolfsii, Sclerotinia spp., Rhizactonia spp., Thielaviopsis basicola and Fusarium spp. (Maciag et al., 2023). Eventually, many more microbial consortia-based formulations came into market, some of them are mentioned in Table 4.
Table 4. Some of the microbial consortia-based products registered and marketed worldwide (Nunes et al., 2024; Maciag et al., 2023)
|
Product
|
Active substance
|
Target disease
|
Country – company/ distributor
|
|
Fertimax
|
Trichoderma viride and
Trichoderma harzianum
|
Pythium, Rhizoctonia solani, Fusarium, Botrytis cinerea, Sclerotium rolfsii, Sclerotinia homoeocarpa and Ustilago tritici
|
India - Skymax Crop Science
|
|
Poabs Green
|
T. viride and T. harzianum
|
Phytophthora capsici, Pythium, Fusarium oxysporum, R. solani, Rosellinia arcuata and nematodes
|
India - Poabs Biotech
|
|
Shakti
|
T. harzianum, T. viride and P. lilacinus
|
Wilt, damping off, Ganoderma and nematodes
|
India - Nivshakti
|
|
Annapurn
|
Trichoderma, Azotobacter,
Azospirillum, Rhizobium and
Pseudomonas
|
Soilborne pathogens
|
India - Multiplex Group
|
|
Anoka
|
T. viride and P. fluorescens
|
Pythium, Phytophthora, Rhizoctonia and Fusarium
|
India - KN Bio Sciences
|
|
Ayush
|
T. viride, P. fluorescens and Micorryzes
|
Sigatoka, panama disease, nematodes, soilborne pathogens and rust
|
India - Bio Sciences
|
|
Trichonativa
|
T. harzianum, T. virens and
Trichoderma parceramosum
|
Botrytis, Alternaria, Venturia, Phytophthora, Fusicoccum, Verticillium, Sclerotinia and Chondrostereum purpureum
|
Chile - BioInsumos Nativa
|
|
3 TAC
|
T. viride, T. harzianum and
Trichoderma longibrachiatum
|
Fusarium, Pythium, Alternaria, Botrytis, Erysiphe, Sclerotinia, Bremis, Phytophthora, Septoria, Cercospora, Leiveillula taurica and P. syringae
|
Chile and Peru - Pinturas Renner, Avance Biotechnologies
|
|
Trichonativa
|
T. viride, T. harzianum and
T. longibrachiatum
|
P. capsici, Pythium, R. solani, Sclerotinia and B. cinerea
|
Chile - BioInsumos Nativa
|
|
Triconova
|
T. harzianum, T. koningiopsis and
B. subtilis
|
F. oxysporum and B. cinerea
|
Peru - Novagri
|
|
Tricox
|
T. koningii and T. harzianum
|
Meloidogyne incognita and Phythophtora capsici
|
Peru - JH Biotech
|
|
Binab T
|
T. polysporum IMI 206039 and T. harzianum IMI 206040
|
Chondrostereum, Verticillium, Rhizoctonia, Fusarium, Phomopsis, Sclerotium, Sclerotinia, Pythium, Botrytis, Heterobasidium and A. mellea
|
UE and USA - Binab Bio Innovation
|
|
Product
|
Active substance
|
Target disease
|
Country – company/ distributor
|
|
Trichogel
|
T. harzianum and T. koningii
|
R. solani
|
Colombia - Soluciones Microbianas
|
|
Tribiol
|
T. harzianum, T. koningii and T. viride
|
R. solani
|
Colombia - Bioproteccion
|
|
Fitotripen
|
T. harzianum, T. koningii and T. viride
|
R. solani, Phytophthora, Fusarium
|
Colombia - Natural Control
|
|
Trich-A-Soil
|
T. harzianum and T. viride
|
Fusarium, Pythium spinosum
|
Australia - Organic Crop Protectants
|
|
Tropimezcla
|
Trichoderma, B. bassiana,
M. anisopliae, P. lilacinus,
S. cereviseae
|
Alternaria
|
Colombia - Soluciones Microbianas
|
|
Condor
|
T. atroviride, Glomus spp. and
rhizosphere bacteria
|
Alternaria, Armillaria, Botrytis, Colletotrichum, Fusarium, Phytophthora, Pyrenochaeta, Pythium, Rhizoctonia, Sclerotinia, Xanthomonas
|
UK - Italpollina
|
|
TNC Tricorr
|
T. hamatum, T. harzianum, T. koningii, T. longibrachiatum, T. reesei, B. subtilis, B. amyloliquefaciens and B. licheniformis
|
Botrytis, Pythium, Fusarium
|
UK - Nutrient Company
|
|
XEDAVIR, PATRIOT GOLD, BIOTRIX, XEDAVIR PFNPE
|
Trichoderma asperellum ICC012 + T25 +
TV1
|
Pythium spp., Phytophthora capsici,
Rhizoctonia solani
|
Xeda International S.A,
Timac AGRO Espana SA
|
|
Binab TF WP,
Binab T Vector
|
Trichoderma atroviride IMI 206040 +
T11
|
Botrytis cinerea, Chondrostereum purpureum
|
Borregaard
Bioplant
|
|
Tusal
|
Trichoderma asperellum T25 +
T. atroviride T11
|
Phytophthora cactorum, Rhizoctonia solani, Sclerotinia sclerotiorum, Phytophthora spp.,
Fusarium spp., Pythium spp., Phomopsis sp.,
|
Newbiotechnic S.A.
|
|
RootShield® PLUS
WP
|
Trichoderma virens G-41 + T. harzianum
Rifai T-22
|
Phytophthora, Rhizoctonia, Pythium, Fusarium,
Thielaviopsis, Cylindrocladium
|
EU, USA and Canada -BioWorks
|
|
BLOSSOM PROTECT,
BONI PROTECT, BOTECTOR
|
Aureobasidium pullulans DSM 14940 +
DSM 14941
|
Fire blight Erwinia amylovora
|
Bio-ferm Biotechnologische
Entwicklung und Produktion GmbH
|
|
Tellus; Foretryx, Bio-Tam2.0, DonJon, Bioten WP, Blindar, Remedier
|
Trichoderma asperellum ICC012 +
T. gamsii ICC080
|
Verticillium dahliae, Rhizoctonia solani, Sclerotinia sclerotiorum, Thielaviopsis basicola, Phytophthora capsici
|
Syngenta, Isagro S p A, Bayer, Gowan
|
CHALLENGES IN FORMULATING AND COMMERCIALIZATION OF MICROBIAL CONSORTIA
Despite the fact, that BCA consortia exhibit higher efficacy and have several advantages over single strain there are several drawbacks which have to be tackled. Compatibility between selected strains is crucial factor in determining the efficacy of consortia. Complex interactions take place between microbes and between plants and microbes, hence interaction between strains has to be assessed before formulating a product in order to have synergistic effect. Xu et al. (2011) found that out of 465 published treatments of microbial consortia, only 2% was found to have synergistic effect. In an incompatible mixture, antibiotic produced by one bio-control strain can diminish the antagonistic effect of other strain in a consortium. Stockwell et al. (2011) reported that extracellular protease produced by Pseudomonas fluorescens A506 supressed antagonistic ability of Pantoea vagans strain C9-1 and Pantoea agglomerans strain Eh252 against fire blight of pear. Further, certain BCA consortia were not proved to be efficient over single inoculation of BCA. Walker et al. (2012) reported that there was no additive effect with co- inoculation of Pseudomonas, Azospirillum and Glomus on secondary metabolite production in maize and the result was similar to that of inoculation with Glomus alone. These negative impacts of incompatibility between strains in consortia emphasizes importance of assessing compatibility of strains for formulating BCA consortia. Development of stable formulations having longer shelf life enhances stable performances of consortia across diverse field conditions. Release of synthetic microbial communities raises ethical issues and thus poses challenge in addressing public conception (Nunes et al., 2024). In spite of progressive research in the field of BCA consortia, there are few BCA consortia-based products due to concerns related to safety, complications in registration of multiple microbial based products, difficulty in determining interaction between microbes (Maciag et al., 2023).
FUTURE PROSPECTS
In recent days, microbial consortia are gaining importance due to their diverse functions like stimulation of plant growth, nutrient acquisition and suppression of plant diseases. Whereas incompatibility among strains and instability in performance still remain as limiting factors. Hence future research should focus on following aspects, understanding complex interactions between microbes, particularly molecular interactions which helps to formulate consortia having synergistic effect. Focus needs to be provided on in vivo assessment of compatibility between strains rather than in vitro assessment. More research is needed on development of stable formulations of consortia exhibiting long term effect and providing stable performance across diverse field conditions. Formulating microbial consortia equally effective in mitigating abiotic stress tolerance along with disease suppression needs to be given attention. Metabolomic profiling and identification of any novel antibiotic production by consortia has to be accelerated. Large number of untapped microbial communities possessing bio-control activity has to be explored. Creating awareness among farmers about the potentiality of bio-control agent based consortia and their diverse functions can replace or reduce chemical pesticide usage in long run.
SUMMARY AND CONCLUSION
Extensive use of chemicals has raised concerns on environmental hazards and health impact among consumers due their residual effect. So, shift from chemical pesticides to eco-friendly bio-control approaches is the need of the hour for the management of diseases. Use of single bio-control agent may exhibit inconsistent performance under field conditions which can be overcome by usage of consortia of bio-control agents which exhibits varied mechanisms of action and have stable performances. In addition to having antagonistic effect against pathogens, bio-control consortia are effective in enhancing crop yield, by exhibiting plant growth promoting traits.
However, challenges like incompatible interactions and regulatory issues need to be addressed for their effective implementation. Bridging gap between scientific research and industry, collaboration between interdisciplinary subjects can further ease the process of adoption of bio-control agent consortia in agriculture practices. Overcoming all these challenges, full potentiality of microbial consortia can be harnessed significantly contributing to sustainable agriculture production.
REFERENCES
Abdeljalil N O B, Vallance J, Gerbore, J, Yacoub A, Daami-Remadi M and Rey P, 2021, Combining potential oomycete and bacterial biocontrol agents as a tool to fight tomato Rhizoctonia root rot. Biological Control, 155: 104521.
Akhtar N, Naveed M, Khalid M, Ahmad N, Rizwan M and Siddique S, 2018, Effect of bacterial consortia on growth and yield of maize grown in Fusarium infested soil. Soil & Environment, 37(1).
Ankati S, Srinivas V, Pratyusha S and Gopalakrishnan S, 2021, Streptomyces consortia-mediated plant defense against Fusarium wilt and plant growth-promotion in chickpea. Microbial Pathogenesis, 157: 104961.
Anshu A, Agarwal P, Mishra K, Yadav U, Verma I, Chauhan S, Srivastava P K and Singh P C, 2022, Synergistic action of Trichoderma koningiopsis and T. asperellum mitigates salt stress in paddy. Physiology and Molecular Biology of Plants, 28(5): 987-1004.
Bo T, Kong C, Zou S, Mo M and Liu Y, 2022, Bacillus nematocida B16 enhanced the rhizosphere colonization of Pochonia chlamydosporia ZK7 and controlled the efficacy of the root-knot nematode Meloidogyne incognita. Microorganisms, 10(2): 218.
Bora P, Bora L C, Bhuyan R P, Hashem A and Abd-Allah E F, 2022, Bioagent consortia assisted suppression in grey blight disease with enhanced leaf nutrients and biochemical properties of tea (Camellia sinensis). Biological Control, 170: 104907.
Brakhage A A, 2013, Regulation of fungal secondary metabolism. Nature Reviews Microbiology, 11(1): 21-32.
Chen Z, Xie Y, Qiu S, Li M and Ge S, 2024, Enriched functional exoproteins and increased exopolysaccharides with altered molecular conformation mutually promoted indigenous microalgal-bacterial consortium biofilm growth under high light intensity. Chemical Engineering Journal, 480: 148056.
Cortes-Tolalpa L, Norder J, van Elsas J D and Falcao Salles J, 2018, Halotolerant microbial consortia able to degrade highly recalcitrant plant biomass substrate. Applied microbiology and biotechnology, 102: 2913-2927.
Devi R, Kaur T, Kour D, Yadav A N and Suman A, 2022, Potential applications of mineral solubilizing rhizospheric and nitrogen fixing endophytic bacteria as microbial consortium for the growth promotion of chilli (Capsicum annum L.). Biologia, 77(10): 2933-2943.
Devi N O, Tombisana Devi R K, Debbarma M, Hajong M and Thokchom S, 2022, Effect of endophytic Bacillus and arbuscular mycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. Egyptian Journal of Biological Pest Control, 32: 1-14.
Dukare A S, Prasanna R, Dubey S C, Nain L, Chaudhary V, Singh R and Saxena A K, 2011, Evaluating novel microbe amended composts as biocontrol agents in tomato. Crop protection, 30(4): 436-442.
Gao H, Niu J, Yang H, Lu Z, Zhou L, Meng F, Lu F and Chen M, 2021, Epimerization of deoxynivalenol by the Devosia strain A6-243 assisted by pyrroloquinoline quinone. Toxins, 14(1): 16.
Garbeva P, Hordijk C, Gerards S and De Boer W, 2014, Volatile-mediated interactions between phylogenetically different soil bacteria. Frontiers in microbiology, 5: 289.
Guo Q, Shi M, Chen L, Zhou J, Zhang L, Li Y, Xue Q and Lai H, 2020, The biocontrol agent Streptomyces pactum increases Pseudomonas koreensis populations in the rhizosphere by enhancing chemotaxis and biofilm formation. Soil Biology and Biochemistry, 144: 107755.
Hao D, Lang B, Wang Y, Wang X, Liu T and Chen J, 2022, Designing synthetic consortia of Trichoderma strains that improve antagonistic activities against pathogens and cucumber seedling growth. Microbial Cell Factories, 21(1): 234.
Hegde G M and Vijaykumar K N, 2022, Formulation, Application and Commercialization of Biopesticides in India Formulation, Application and Commercialization of Biopesticides in India. Asia Pacific Biofertilizers and Biopesticides Information Platform.
Huang W, Chang J, Wang P, Liu C, Yin Q, Zhu Q, Lu F and Gao T, 2018, Effect of the combined compound probiotics with mycotoxin degradation enzyme on detoxifying aflatoxin B1 and zearalenone. The Journal of toxicological sciences, 43(6): 377-385.
Jadav P C, Hegde G M, Jahagirdar S and Navi V, 2022, Evaluation of microbial consortia against Sclerotium rolfsii Sacc. causing foot rot of wheat. Journal of Farm Sciences, 35(02): 219-222.
Jain A, Singh A, Singh S and Singh H B, 2015, Phenols enhancement effect of microbial consortium in pea plants restrains Sclerotinia sclerotiorum. Biological Control, 89: 23-32.
Jariyal M, Jindal V, Mandal K, Gupta V K and Singh B, 2018, Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotoxicology and environmental safety, 159: 310-316.
Johnson I, Ramjegathesh R, Sheela J, Shoba N and Maheshwarappa H P, 2017, Development of microbial consortia for the management of leaf blight disease of coconut. Acta Phytopathologica et Entomologica Hungarica, 52(1): 1-14.
Kaur T, Devi R, Kumar S, Sheikh I, Kour D and Yadav A N, 2022, Microbial consortium with nitrogen fixing and mineral solubilizing attributes for growth of barley (Hordeum vulgare L.). Heliyon, 8(4).
Kaur T, Devi R, Kumar S, Kour D and Yadav A N, 2023, Plant growth promotion of pearl millet (Pennisetum glaucum L.) by novel bacterial consortium with multifunctional attributes. Biologia
78(2): 621-631.
Khan B A, Nadeem M A, Nawaz H, Amin M M, Abbasi G H, Nadeem M, Ali M, Ameen M, Javaid M M, Maqbool R and Ikram M, 2023, Pesticides: impacts on agriculture productivity, environment, and management strategies. In Emerging contaminants and plants: Interactions, adaptations and remediation technologies (pp. 109-134). Cham: Springer International Publishing.
Kumar S M, Chowdappa P and Krishna V, 2015, Development of seed coating formulation using consortium of Bacillus subtilis OTPB1 and Trichoderma harzianum OTPB3 for plant growth promotion and induction of systemic resistance in field and horticultural crops. Indian Phytopathology 68(1): 25-31.
Kumar K H and Jagadeesh K S, 2016, Microbial consortia-mediated plant defense against phytopathogens and growth benefits. South Indian Journal of Biological Sciences, 2(4): 395-403.
Kumar S, Chandra R, Behera L, Sudhir I, Meena M, Singh S and Keswani C, 2023, Microbial consortium mediated acceleration of the defense response in potato against Alternaria solani through prodigious inflation in phenylpropanoid derivatives and redox homeostasis. Heliyon, 9(11).
Lashani E, Amoozegar M A, Turner R J. and Moghimi H, 2023, Use of microbial consortia in bioremediation of metalloid polluted environments. Microorganisms, 11(4): 891.
Lutz M P, Wenger S, Maurhofer M, Défago G and Duffy B, 2004, Signaling between bacterial and fungal biocontrol agents in a strain mixture. FEMS microbiology ecology, 48(3): 447-455.
Maciag T, Kozieł E, Rusin P, Otulak-Kozieł K, Jafra S and Czajkowski R, 2023, Microbial consortia for plant protection against diseases: more than the sum of its parts. International Journal of Molecular Sciences, 24(15): 12227.
Maheshwari D K, Dubey R C, Agarwal M, Dheeman S, Aeron A and Bajpai V K, 2015, Carrier based formulations of biocoenotic consortia of disease suppressive Pseudomonas aeruginosa KRP1 and Bacillus licheniformis KRB1. Ecological Engineering, 81: 272-277.
Minchev Z, Kostenko O, Soler R and Pozo M J, 2021, Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Frontiers in Plant Science, 12: 756368.
Mondal A, Mahapatra S, Chakraborty S, Debnath D, Das T and Samanta M, 2024, Eco-friendly Management of Collar Rot of Lentil by Introduced Native Rhizobacterial Candidates. Indian Journal of Agricultural Research, 58(1).
Niu B, Wang W, Yuan Z, Sederoff R R, Sederoff H, Chiang V L and Borriss R, 2020, Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Frontiers in Microbiology, 11: 585404.
Nunes P S, Junior G V L, Mascarin G M, Guimaraes R A, Medeiros F H, Arthurs S and Bettiol W, 2024, Microbial consortium of biological products: do they have a future?. Biological Control,: 105439.
Nurcahyanti S D, Masnilah R, Budiman S A, Tanzil A I and Hendra Nurdika A A, 2024, Microbial consortium formulation in liquid organic fertilizer for managing bacterial leaf blight (Xanthomonas oryzae pv. oryzae), rice blast (Pyricularia oryzae), and enhancing rice productivity. Biodiversitas: Journal of Biological Diversity, 25(5).
Palmieri D, Vitullo D, De Curtis F and Lima G, 2017, A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant and soil, 412: 425-439.
Palmieri D, Ianiri G, Del Grosso C, Barone G, De Curtis F, Castoria R and Lima G, 2022, Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae, 8(7): 577.
Pishchany G, Mevers E, Ndousse-Fetter S, Horvath Jr D J, Paludo C R, Silva-Junior E A, Koren S, Skaar E P, Clardy J and Kolter R, 2018, Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proceedings of the National Academy of Sciences, 115(40): 10124-10129.
Poveda J and Eugui D, 2022, Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biological Control, 176: 105100.
Ram R M, Tripathi R, Birla H, Dilnashin H, Singh S P and Keswani C, 2019, Mixed PGPR consortium: an effective modulator of antioxidant network for management of collar rot in cauliflower. Archives of Phytopathology and Plant Protection, 52(7-8): 844-862.
Resti Z, Liswarni Y and Martinius M, 2020, Endophytic bacterial consortia as biological control of bacterial leaf blight and plant growth promoter of rice (Oryza sativa L.). Journal of Applied Agricultural Science and Technology, 4(2): 134-145.
Ristaino J B, Anderson P K, Bebber D P, Brauman K A, Cunniffe N J, Fedoroff N V, Finegold C, Garrett K A, Gilligan C A, Jones C M and Martin M D, 2021, The persistent threat of emerging plant disease pandemics to global food security. Proceedings of the National Academy of Sciences, 118(23): 2022239118.
Santoyo G, Guzmán-Guzmán P, Parra-Cota F I, Santos-Villalobos S D L, Orozco-Mosqueda, M D C and Glick B R, 2021, Plant growth stimulation by microbial consortia. Agronomy, 11(2): 219.
Sanzani S M, Reverberi M and Geisen R, 2016, Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biology and Technology, 122: 95-105.
Saxena A, Mishra S, Ray S, Raghuwanshi R and Singh H B, 2020, Differential reprogramming of defense network in Capsicum annum L. plants against colletotrichum truncatum infection by phyllospheric and rhizospheric trichoderma strains. Journal of plant growth regulation, 39: 751-763.
Singh A, Sarma B K, Upadhyay R S and Singh H B, 2013, Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiological Research, 168(1): 33-40.
Singh D, Jadon K S, Verma A, Geat N, Sharma R, Meena K K and Kakani R K, 2024, Formulations of synergistic microbial consortia for enhanced systemic resistance against Fusarium wilt in cumin. International Microbiology: 1-27.
Sood D, Sharma A and Sharma M, 2021, Management of bacterial wilt of tomato through consortium of biocontrol agents. Journal of Pharmacognosy and Phytochemistry, 10(1): 1355-1358.
Stockwell V O, Johnson K B, Sugar D and Loper J E, 2011, Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology, 101(1): 113-123.
Taheur F B, Fedhila K, Chaieb K, Kouidhi B, Bakhrouf A and Abrunhosa L, 2017, Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. International journal of food microbiology, 251: 1-7.
Thakkar A and Saraf M, 2015, Development of microbial consortia as a biocontrol agent for effective management of fungal diseases in Glycine max L. Archives of Phytopathology and Plant Protection, 48(6): 459-474.
Thomloudi E E, Tsalgatidou P C, Douka D, Spantidos T N, Dimou M, Venieraki A and Katinakis P, 2019, Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hellenic Plant Protection Journal, 12(2): 61-77.
Trivedi S, Srivastava M, Ratan V, Mishra A, Dixit S and Pandey S, 2020, Evaluation of microbial consortia on systemic resistance against chickpea wilt. Bangladesh Journal of Botany, 49(3): 653-661.
Vespermann A, Kai M and Piechulla B, 2007, Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Applied and environmental microbiology, 73(17): 5639-5641.
Vishwakarma K, Kumar N, Shandilya C, Mohapatra S, Bhayana S and Varma A, 2020, Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Frontiers in Microbiology, 11: 560406.
Walker V, Couillerot O, Von Felten A, Bellvert F, Jansa J, Maurhofer M, Bally R, Moënne-Loccoz Y and Comte G, 2012, Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant and soil, 356: 151-163.
Wang, Yanxia, Gang Wang, Yijun Dai, Yu Wang, Yin-Won Lee, Jianrong Shi, and Jianhong Xu, 2020, "Biodegradation of deoxynivalenol by a novel microbial consortium." Frontiers in microbiology, 10: 2964.
Wang Y, Hu J, Dai Y, Wang Y, Shi J, Wang G, Xu J and De Saeger S, 2023, Design and characterization of an artificial two-strain bacterial consortium for the efficient biodegradation of deoxynivalenol. Biological Control, 179: 105172.
Wang Y, Yang L, Xu J, Xin F and Jiang L, 2023, Applications of synthetic microbial consortia in biological control of mycotoxins and fungi. Current Opinion in Food Science: 101074.
Wong C K F, Saidi N B, Vadamalai G, Teh C Y and Zulperi D, 2019, Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against Fusarium wilt of banana. Journal of applied microbiology, 127(2): 544-555.
Wu D, Wang W, Yao Y, Li H, Wang Q and Niu B, 2023, Microbial interactions within beneficial consortia promote soil health. Science of the Total Environment: 165801.
Xu X, Qin G and Tian S, 2008, Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. International Journal of Food Microbiology, 126(1-2): 153-158.
Xu X M, Jeffries P, Pautasso M and Jeger M J, 2011, Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology, 101(9): 1024-1031.
Zhai Y, Zhong L, Gao H, Lu Z, Bie X, Zhao H, Zhang C and Lu F, 2019, Detoxification of deoxynivalenol by a mixed culture of soil bacteria with 3-epi-deoxynivalenol as the main intermediate. Frontiers in microbiology, 10: 2172.
Zhang J, Ahmed W, Dai Z, Zhou X, He Z, Wei L and Ji G, 2022, Microbial consortia: An engineering tool to suppress clubroot of Chinese cabbage by changing the rhizosphere bacterial community composition. Biology, 11(6): 918.
Challenges and Potentials of Microbial Consortia for Plant Disease Management and Sustainable Productivity
DOI: https://doi.org/10.56669/LRKM6748
ABSTRACT
Biological control offers a promising and safer approach to protect plants from pathogens. Bio-control agents provide effective control of diseases by adopting various strategies like competition, antibiosis, hyperparasitism etc. But they exhibit inconsistent performance under diverse field conditions, which demands for the search of novel approaches in biological control of plant diseases. Under such circumstances, microbial consortia which exhibit diverse modes of action and have synergistic effect in enhancing efficacy of bio-control agents can be an effective strategy in combating plant diseases. Microbial consortia in addition to disease suppression, contributes to plant growth promotion, nutrient assimilation and bioremediation. Despite of being versatile in action, microbial consortia face several challenges with respect to incompatibility, regulatory issues which have to be addressed for their effective implementation in managing diseases. This review focuses on potentiality of microbial consortia over single inoculants in managing diseases, microbial interactions within consortia, mechanism of action, challenges in formulation and commercialization of microbial consortia.
Keywords: Biological control, Diseases, Efficacy, Microbial consortia.
INTRODUCTION
Global food demand is increasing rapidly, whereas agricultural production is facing many uncertainties such as climate change, abiotic stresses and biotic stresses such as insects, diseases and nematodes. Plant diseases pose serious threat to global food production, as evidenced by historical pandemics such as Irish famine and Great Bengal famine (Ristaino et al., 2021). Chemical pesticides have been used extensively to manage diseases, but chemical pesticides pose environmental hazards and adversely affects non-target organisms (Khan et al., 2023). Owing to the consumers demand towards low or zero chemical residues and regulatory restrictions on permitted level of chemical residues, biological control agents (BCA) based control of plant diseases are emerging as effective tools for the management of diseases. Different mechanisms of action exhibited by bio-control agents such as competition, antibiosis, mycoparasitism and induction of plant defence play crucial role in sustainable management of diseases (Palmieri et al., 2022). However, single strain inoculation of bio-control agents was not found to be effective under diverse field conditions. The Potentials of bio-control agents can be enhanced using mixtures of different bio-control agents exhibiting synergistic effect (Poveda et al., 2022). Application of microbial consortia improves efficacy and consistency of microbes under diverse environmental conditions. Varied mechanisms of action exhibited by each of bio-control agents in consortia enhances disease suppressiveness of microbial consortia (Kumar and Jagadeesh, 2016). Thus, microbial consortia can be a promising approach for sustainable management of diseases.
MICROBIAL CONSORTIA
Microbial consortia are a diverse group of microbes that co-exist and interact with each other (Lashani et al., 2023). Microbial consortia have diverse roles in agriculture like bioremediation of pesticides (Jariyal et al., 2018), plant biomass degradation (Cortes-Tolalpa et al., 2018), plant growth promotion (Santoyo et al., 2021) and disease suppression (BCA consortia). BCA consortia comprise of different bacterial antagonist or fungal antagonist or both. Consortia comprising of bacterial antagonist Pseudomonas fluorescens (NAIMCC-B-01981), fungal antagonist Trichoderma harzianum (MH027645.1) and endophyte Neofusicoccum parvum exhibited maximum inhibition of Sclerotium rolfsii compared to single antagonist (Jadav et al., 2022). In contrary to a single strain, BCA consortia are consistent under diverse crop conditions. Thus, efficacy of bio-control agents under field conditions can be improved by the usage of a mixture of BCAs, and synergistic effect of different BCAs can be exploited (Hegde and Vijaykumar, 2022).
Microbial consortia have several advantages in plant disease management over the use of a single strain such as i) Consortia of bio-control agents targets wide range of phytopathogens (Thakkar and Saraf, 2015), ii) Improves soil suppressiveness by increasing microbial diversity in soil rhizosphere (Palmieri et al., 2017), iii) Diverse species occupy different ecological niches in rhizosphere and there by restricts competition between them (Thomloudi et al., 2019), iv) Microbe interactions in consortium enhances rhizosphere colonization (Wu et al., 2023), v) Employing multiple strains of microorganisms with diverse modes of action improves bio-control efficiency of consortia (Thakkar and Saraf, 2015).
MICROBIAL INTERACTIONS AND THEIR EFFECT ON DISEASE MANAGEMENT
Microorganisms are ubiquitous and have diverse roles in agriculture by interacting with each other in rhizosphere region and with plants as well in phyllosphere region and contribute to biotic and abiotic stress management. Through their interactions, they can efficiently colonize rhizosphere and certain beneficial plant microbiota can even enhance disease resistance. Thus, microbial interactions have direct as well as indirect influence on the suppression of plant diseases.
Elevated production of antimicrobial substances
Bio-control agents have direct antagonistic effect on pathogens by production of toxic antimicrobial substances like bacteriocins, lipopeptides (iturin, fengycin and surfactin), chitinase and killer toxins. Single strain may produce low concentration of antimicrobial substance, whereas microbe interactions can boost its production to higher level (Brakhage, 2013). 2,4-diacetylphloroglucinol (DAPG) from P. fluorescens strain CHA0 enhanced production of N-acetyl-β-d-glucosaminidase in Trichoderma P1 strain when co-cultured (Lutz et al., 2004). Rhizobacterial isolates comprising of Stenotrophomonas, Serratia, and Bacillus species can produce complex blend of VOC’s and inhibits growth of phytopathogenic fungi (Vespermann et al., 2007). Microbial interactions are also known to produce novel antimicrobial compounds in addition to elevating their production (Niu et al., 2020). A novel antibiotic amycomicin has been identified to be produced by interaction between Amycolatopsis sp. AA4 and Streptomyces coelicolor M145 (Pishchany et al., 2018).
Efficient rhizosphere colonization
Efficient colonization of rhizosphere by bio-control agents acts as first line of defence against soil borne pathogens. Mixture of four bacterial isolates comprising of Serratia marcescens, Pseudomonas fluorescens, Rahnella aquatilis and Bacillus amyloliquefaciens improved soil suppressiveness against Fusarium oxysporum f. sp. ciceris race 0 and Fusarium solani f. sp. pisi causing Fusarium decline in chickpea by increasing microbial diversity in rhizosphere (Palmieri et al., 2017). Garbeva et al. (2014) reported volatile mediated interaction between different soil bacteria as Collimonas pratensis, Serratia plymuthica, Paenibacillus spp. and Pedobacter spp. stimulated the growth of Pseudomonas fluorescens and further C. pratensis triggered, antimicrobial secondary metabolite production in P. fluorescens. Thus, microbial consortia can improve soil health by increasing microbial diversity in rhizosphere.
Synergistic biofilm formation
Biofilm is a community of microorganisms in which cells adhere to each other or also to a surface which are embedded in extracellular matrix. Bio-film formation takes place through cell-to-cell communication in microbes by quorum sensing. Bio-film formation potentially occupies sites that can be invaded by pathogen and thus causes spatial and nutritive competition to pathogens. Chen et al., (2024) reported that altered molecular conformations and increased exoproteins, exopolysaccharides in microalgal-bacterial consortium enhanced biofilm formation. Streptomyces pactum improved bio-film formation, enhanced nutrient utilization, environmental adaptation in Pseudomonas koreensis and boosted its competitiveness (Guo et al., 2020).
Motility
Dispersal of fungal spores on bacteria and bacterial dispersal takes place through fungal mycelium in rhizosphere. The mycelia of Trichoderma can serve as a supporting layer for formation of bacterial biofilms and can aid bacterial migration in the soil (Niu et al., 2020). Motility helps BCA to colonize rhizosphere region and supress soil borne pathogens.
Microbial interactions through their synergistic effect can thus improve efficacy of bio-control agents. Diverse group of microorganisms can deplete resources rapidly than single strain alone and causes competitive inhibition of pathogens. In addition, microbes exhibit syntrophic interaction (cross feeding), wherein both microbes together carry out metabolism of single complex substrate which neither one can do alone. Thus, syntrophic interaction can enhance survival of BCA by efficient utilization of nutrients in its vicinity.
MODE OF ACTION
Bio-control agents have direct as well as indirect antagonistic mechanisms against pathogens like hyperparasitism, antibiosis, competition for space and nutrients, production of volatile organic compounds, toxin production and inducing systemic resistance in plants. BCA consortia may exhibit diverse mode of action and are consistent in action compared to single strain of BCA. Mode of action of certain microbial consortia are shown in Table 1.
MICROBIAL CONSORTIA AND THEIR ROLE IN ENHANCING PLANT RESISTANCE
Plants for their better acclimatization for biotic and abiotic stresses have evolved into various mechanisms. When attacked by pathogens, plants activate their defence responses rapidly to protect themselves. Induction of host resistance is indirect mechanism of action exhibited by BCA. Exogenous application of BCA elicits defence responses in plants like production of phenolic compounds, antioxidant enzymes, pathogenesis related enzymes and induction of systemic resistance. Whereas, priming with several beneficial microorganisms can elevate extent and duration of activation of defence responses.
Production of antioxidant enzymes
Plants in response to attack by pathogens as an immediate response, leads to rapid accumulation of reactive oxygen species (ROS). When produced in controlled manner they are involved in signal transduction but abruptive production of ROS can cause disruption of normal metabolism and key cellular functions. BCA can regulate ROS levels by production of antioxidant enzymes like superoxide dismutase (SOD) and peroxidase (POD) and provides protection against diverse plant pathogens (Xu et al., 2008). Ram et al. (2019) showed that consortium comprising of Trichoderma harzianum TNHU27 and Pseudomonas aeruginosa PJHU15 were effective in regulating antioxidant activity in cauliflower against Sclerotinia sclerotiorum causing collar rot in cauliflower and was superior to single BCA inoculation by induction of enhanced and faster defence response. Singh et al. (2013) stated rhizospheric microbial consortium consisting of fluorescent Pseudomonas (PHU094), Trichoderma (THU0816) and Rhizobium (RL091) strain increased phenylalanine ammonia lyase, polyphenol oxidase, accumulation of total phenol content in chickpea against Sclerotium rolfsii causing collar rot in triple microbial consortium as compared to dual microbial consortium and single microbe inoculation.
Activation of systemic resistance
Plants in response to attack by pathogens shows hypersensitive response which further induces systemic acquired resistance (SAR) mediated by salicyclic acid signalling leading to accumulation of PR proteins having antimicrobial activities. On the other hand, beneficial rhizobacteria triggers induced systemic resistance (ISR) mediated by Jasmonic acid (JA) and ethylene. Both SAR and ISR are effective against wide range of pathogens.
Bacillus amyloliquefaciens CECT 8238 and Rhizophagus irregularis consortia were able to induce ISR against Botrytis cinerea in tomato by reducing the area of the necrotic lesions by 33-37% (Minchev et al., 2021). Seed treatment with consortia comprising of 1% Trichoderma harzianum + 2% Pseudomonas fluorescens + 2% Bacillus subtilis induced 1.4%, 1.6%, 2.3% increase in peroxidase, polyphenol oxidase and total phenol in chickpea respectively and showed elevated production of defence related enzymes as compared to single BCA application (Trivedi et al., 2020). Activation of defence response was observed in potato plants for protection against early blight caused by Alternaria solani by increased accumulation of pathogenesis related proteins like chitinase and β-1,3-glucanase in triple microbial consortia compared to other treatments (Kumar et al., 2023).
Production of Phenolic compounds
Phenolic compounds are group of metabolites that can alter various physiological process in plants. Phenols participate in lignin deposition and act as mechanical barrier for penetration of pathogens. Bacillus subtilis BHHU100, Pseudomonas aeruginosa PJHU15 and Trichoderma harzianum TNHU27 increased accumulation of phenolics like chlorogenic acid, p-coumaric acid, syringic acid, cinnamic acid, gallic acid, shikimic acid in collar and leaf region and enhanced protection against Sclerotinia sclerotiorum in pea (Jain et al., 2015). Two strains of Trichoderma viz., phyllosphere isolate BHUF4 and rhizospheric isolate T16A were effective in protecting chilli plants from infection of Colletotrichum truncatum by accumulation of phenolics like kaempeferol (6.22-fold), capsaicin (16.1-fold), quercetin (5.36-fold) and salicylic acid (94.88-fold) (Saxena et al., 2020).
MICROBIAL CONSORTIA IN DETOXIFICATION OF MYCOTOXINS
Mycotoxins are toxic metabolites produced by certain molds or fungi that can contaminate food and feed and pose significant health risks to humans and animals, ranging from acute poisoning to chronic diseases. Some of the common mycotoxins produced in grains and nuts are aflatoxins, ochratoxins, fumonisins, zearalenone (ZEN) and deoxynivalenol (DON). Significant portion of postharvest losses is due to fungal diseases and mycotoxin production. The role of mycotoxins in the progression of diseases suggests that reduction of mycotoxins can be a valuable approach for management of diseases. Use of fungicides is not desirable as it can have residual effect and hence use of microbes for detoxification is a promising approach. (Sanzani et al., 2016).
Synthetic microbial consortia exhibit greater efficiency in detoxifying mycotoxins compared to monocultures. Synergistic interactions among various species within microbial consortia promotes specific catalytic reactions leading to efficient mycotoxin detoxification. Concurrent transformation of diverse mycotoxins remains difficult for individual strains because of the chemical diversity of mycotoxins. However, using a combination of different microbes, each capable of performing distinct functions, offers a better strategy to reduce the risk of contamination by multiple mycotoxins. The effectiveness of surface adsorption of mycotoxins can be significantly improved through microbial consortia (Wang et al., 2023). In recent days, substantial research has focused on the detoxification of mycotoxins using microbial consortia and some of them are detailed in Table 2.
FORMULATIONS AND APPLICATION METHODS
Different application methods of microbial consortia such as seed treatment, foliar application and soil application are proved to be effective in managing diseases. Despite of having high potentiality in suppressing disease, formulations and application methods plays crucial role in determining their efficacy. Composts when amended with Bacillus subtillis B5, Anabaena oscillarioides C12 and compost tea preparations were effective in reducing severity of root diseases of tomato caused by Rhizoctonia solani, Pythium aphanidermatum, Fusarium oxysporum and Pythium debaryanum (Dukare et al., 2011). Kumar et al. (2015) developed seed coating formulations of B. subtilis OTPB1 + T. harzianum (OTPB3) using vermicompost has carrier material and found that microbial consortia formulation upregulated defence related enzymes such as superoxide dismutase, peroxidase and polyphenol oxidase and reduced lesion size caused by Alternaria solani and Phytophthora infestans in tomato. Consortia comprising of B. subtilis (Km1), P. fluorescens Pf1 and T. viride (TNAU) through soil application using talc as carrier material along with farmyard manure was proved to be effective in managing leaf blight of coconut caused by Lasiodiplodia theobromae (Johnson et al., 2017). Maheshwari et al. (2015) proved that vermiculite based consortial bioformulation consisting of Bacillus licheniformis KRB1 and Pseudomonas aeruginosa KRP1 had higher viability of bacteria and was antagonistic against Fusarium oxysporum and Sclerotinia sclerotiorum. Pesta granules application of consortium of Pseudomonas aeruginosa DRB1 and Trichoderma harzianum CBF2 reduced disease severity of fusarium wilt of banana by 66.67% (Wong et al., 2019). Drenching of Trichoderma viride + Bacillus subtilis + Pseudomonas flourescens consortia along with neem cake and molasses was highly effective in controlling bacterial wilt of tomato caused by Ralstonia solanacearum and disease incidence was not observed in treated plot (Sood et al., 2021). Liquid organic fertilizers formulated using cattle manure, rice straw, vegetable and fruit waste enriched with consortium of Trichoderma sp., Pseudomonas putida Pf10 and Bacillus subtilis JB12 when applied through combination of all three application methods viz., seed treatment, seedling dip and foliar spray reduced disease severity of bacterial leaf blight by 21% and blast of rice by 4% (Nurcahyanti et al., 2024).
MICROBIAL CONSORTIA FOR SUSTAINABLE AGRICULTURAL PRODUCTION
Plant growth promoting rhizobacteria (PGPRs) and their interaction with plants have tremendous role in sustainable agricultural production. They have diverse roles like plant growth promotion, mitigation of abiotic stresses like salt and drought tolerance, siderophore production and nutrient solubilisation. Plant growth promotion is attributed to their ability to produce gibberellins, cytokinins, auxins and ethylene. Ethylene apart from stimulating plant growth, is involved in defence signalling and inducing resistance in plants. Nutrient assimilation is important trait exhibited by microbes like N2 fixation by Rhizobia species through symbiotic relationship with plants and siderophore production which makes iron available to plants (Vishwakarma et al., 2020). Some of the multifunctionality traits of microbial consortia are presented in Table 3.
COMMERCIAL FORMULATIONS OF MICROBIAL CONSORTIA
Owing to many advantages of microbial BCA consortia like consistency, longevity, modularity, acclimatization to diverse environmental conditions and its capacity to target a wide range of pathogens, formulations comprising of multiple BCA are gaining importance. Bio-control product based on microbial consortia (BIO-TAM 2.0) comprising of Trichoderma asperellum and T. gamsii was first registered in 2015 and was known to be effective against Verticillium spp., Phytophthora spp., Pythium spp., Sclerotium rolfsii, Sclerotinia spp., Rhizactonia spp., Thielaviopsis basicola and Fusarium spp. (Maciag et al., 2023). Eventually, many more microbial consortia-based formulations came into market, some of them are mentioned in Table 4.
Table 4. Some of the microbial consortia-based products registered and marketed worldwide (Nunes et al., 2024; Maciag et al., 2023)
Product
Active substance
Target disease
Country – company/ distributor
Fertimax
Trichoderma viride and
Trichoderma harzianum
Pythium, Rhizoctonia solani, Fusarium, Botrytis cinerea, Sclerotium rolfsii, Sclerotinia homoeocarpa and Ustilago tritici
India - Skymax Crop Science
Poabs Green
T. viride and T. harzianum
Phytophthora capsici, Pythium, Fusarium oxysporum, R. solani, Rosellinia arcuata and nematodes
India - Poabs Biotech
Shakti
T. harzianum, T. viride and P. lilacinus
Wilt, damping off, Ganoderma and nematodes
India - Nivshakti
Annapurn
Trichoderma, Azotobacter,
Azospirillum, Rhizobium and
Pseudomonas
Soilborne pathogens
India - Multiplex Group
Anoka
T. viride and P. fluorescens
Pythium, Phytophthora, Rhizoctonia and Fusarium
India - KN Bio Sciences
Ayush
T. viride, P. fluorescens and Micorryzes
Sigatoka, panama disease, nematodes, soilborne pathogens and rust
India - Bio Sciences
Trichonativa
T. harzianum, T. virens and
Trichoderma parceramosum
Botrytis, Alternaria, Venturia, Phytophthora, Fusicoccum, Verticillium, Sclerotinia and Chondrostereum purpureum
Chile - BioInsumos Nativa
3 TAC
T. viride, T. harzianum and
Trichoderma longibrachiatum
Fusarium, Pythium, Alternaria, Botrytis, Erysiphe, Sclerotinia, Bremis, Phytophthora, Septoria, Cercospora, Leiveillula taurica and P. syringae
Chile and Peru - Pinturas Renner, Avance Biotechnologies
Trichonativa
T. viride, T. harzianum and
T. longibrachiatum
P. capsici, Pythium, R. solani, Sclerotinia and B. cinerea
Chile - BioInsumos Nativa
Triconova
T. harzianum, T. koningiopsis and
B. subtilis
F. oxysporum and B. cinerea
Peru - Novagri
Tricox
T. koningii and T. harzianum
Meloidogyne incognita and Phythophtora capsici
Peru - JH Biotech
Binab T
T. polysporum IMI 206039 and T. harzianum IMI 206040
Chondrostereum, Verticillium, Rhizoctonia, Fusarium, Phomopsis, Sclerotium, Sclerotinia, Pythium, Botrytis, Heterobasidium and A. mellea
UE and USA - Binab Bio Innovation
Product
Active substance
Target disease
Country – company/ distributor
Trichogel
T. harzianum and T. koningii
R. solani
Colombia - Soluciones Microbianas
Tribiol
T. harzianum, T. koningii and T. viride
R. solani
Colombia - Bioproteccion
Fitotripen
T. harzianum, T. koningii and T. viride
R. solani, Phytophthora, Fusarium
Colombia - Natural Control
Trich-A-Soil
T. harzianum and T. viride
Fusarium, Pythium spinosum
Australia - Organic Crop Protectants
Tropimezcla
Trichoderma, B. bassiana,
M. anisopliae, P. lilacinus,
S. cereviseae
Alternaria
Colombia - Soluciones Microbianas
Condor
T. atroviride, Glomus spp. and
rhizosphere bacteria
Alternaria, Armillaria, Botrytis, Colletotrichum, Fusarium, Phytophthora, Pyrenochaeta, Pythium, Rhizoctonia, Sclerotinia, Xanthomonas
UK - Italpollina
TNC Tricorr
T. hamatum, T. harzianum, T. koningii, T. longibrachiatum, T. reesei, B. subtilis, B. amyloliquefaciens and B. licheniformis
Botrytis, Pythium, Fusarium
UK - Nutrient Company
XEDAVIR, PATRIOT GOLD, BIOTRIX, XEDAVIR PFNPE
Trichoderma asperellum ICC012 + T25 +
TV1
Pythium spp., Phytophthora capsici,
Rhizoctonia solani
Xeda International S.A,
Timac AGRO Espana SA
Binab TF WP,
Binab T Vector
Trichoderma atroviride IMI 206040 +
T11
Botrytis cinerea, Chondrostereum purpureum
Borregaard
Bioplant
Tusal
Trichoderma asperellum T25 +
T. atroviride T11
Phytophthora cactorum, Rhizoctonia solani, Sclerotinia sclerotiorum, Phytophthora spp.,
Fusarium spp., Pythium spp., Phomopsis sp.,
Newbiotechnic S.A.
RootShield® PLUS
WP
Trichoderma virens G-41 + T. harzianum
Rifai T-22
Phytophthora, Rhizoctonia, Pythium, Fusarium,
Thielaviopsis, Cylindrocladium
EU, USA and Canada -BioWorks
BLOSSOM PROTECT,
BONI PROTECT, BOTECTOR
Aureobasidium pullulans DSM 14940 +
DSM 14941
Fire blight Erwinia amylovora
Bio-ferm Biotechnologische
Entwicklung und Produktion GmbH
Tellus; Foretryx, Bio-Tam2.0, DonJon, Bioten WP, Blindar, Remedier
Trichoderma asperellum ICC012 +
T. gamsii ICC080
Verticillium dahliae, Rhizoctonia solani, Sclerotinia sclerotiorum, Thielaviopsis basicola, Phytophthora capsici
Syngenta, Isagro S p A, Bayer, Gowan
CHALLENGES IN FORMULATING AND COMMERCIALIZATION OF MICROBIAL CONSORTIA
Despite the fact, that BCA consortia exhibit higher efficacy and have several advantages over single strain there are several drawbacks which have to be tackled. Compatibility between selected strains is crucial factor in determining the efficacy of consortia. Complex interactions take place between microbes and between plants and microbes, hence interaction between strains has to be assessed before formulating a product in order to have synergistic effect. Xu et al. (2011) found that out of 465 published treatments of microbial consortia, only 2% was found to have synergistic effect. In an incompatible mixture, antibiotic produced by one bio-control strain can diminish the antagonistic effect of other strain in a consortium. Stockwell et al. (2011) reported that extracellular protease produced by Pseudomonas fluorescens A506 supressed antagonistic ability of Pantoea vagans strain C9-1 and Pantoea agglomerans strain Eh252 against fire blight of pear. Further, certain BCA consortia were not proved to be efficient over single inoculation of BCA. Walker et al. (2012) reported that there was no additive effect with co- inoculation of Pseudomonas, Azospirillum and Glomus on secondary metabolite production in maize and the result was similar to that of inoculation with Glomus alone. These negative impacts of incompatibility between strains in consortia emphasizes importance of assessing compatibility of strains for formulating BCA consortia. Development of stable formulations having longer shelf life enhances stable performances of consortia across diverse field conditions. Release of synthetic microbial communities raises ethical issues and thus poses challenge in addressing public conception (Nunes et al., 2024). In spite of progressive research in the field of BCA consortia, there are few BCA consortia-based products due to concerns related to safety, complications in registration of multiple microbial based products, difficulty in determining interaction between microbes (Maciag et al., 2023).
FUTURE PROSPECTS
In recent days, microbial consortia are gaining importance due to their diverse functions like stimulation of plant growth, nutrient acquisition and suppression of plant diseases. Whereas incompatibility among strains and instability in performance still remain as limiting factors. Hence future research should focus on following aspects, understanding complex interactions between microbes, particularly molecular interactions which helps to formulate consortia having synergistic effect. Focus needs to be provided on in vivo assessment of compatibility between strains rather than in vitro assessment. More research is needed on development of stable formulations of consortia exhibiting long term effect and providing stable performance across diverse field conditions. Formulating microbial consortia equally effective in mitigating abiotic stress tolerance along with disease suppression needs to be given attention. Metabolomic profiling and identification of any novel antibiotic production by consortia has to be accelerated. Large number of untapped microbial communities possessing bio-control activity has to be explored. Creating awareness among farmers about the potentiality of bio-control agent based consortia and their diverse functions can replace or reduce chemical pesticide usage in long run.
SUMMARY AND CONCLUSION
Extensive use of chemicals has raised concerns on environmental hazards and health impact among consumers due their residual effect. So, shift from chemical pesticides to eco-friendly bio-control approaches is the need of the hour for the management of diseases. Use of single bio-control agent may exhibit inconsistent performance under field conditions which can be overcome by usage of consortia of bio-control agents which exhibits varied mechanisms of action and have stable performances. In addition to having antagonistic effect against pathogens, bio-control consortia are effective in enhancing crop yield, by exhibiting plant growth promoting traits.
However, challenges like incompatible interactions and regulatory issues need to be addressed for their effective implementation. Bridging gap between scientific research and industry, collaboration between interdisciplinary subjects can further ease the process of adoption of bio-control agent consortia in agriculture practices. Overcoming all these challenges, full potentiality of microbial consortia can be harnessed significantly contributing to sustainable agriculture production.
REFERENCES
Abdeljalil N O B, Vallance J, Gerbore, J, Yacoub A, Daami-Remadi M and Rey P, 2021, Combining potential oomycete and bacterial biocontrol agents as a tool to fight tomato Rhizoctonia root rot. Biological Control, 155: 104521.
Akhtar N, Naveed M, Khalid M, Ahmad N, Rizwan M and Siddique S, 2018, Effect of bacterial consortia on growth and yield of maize grown in Fusarium infested soil. Soil & Environment, 37(1).
Ankati S, Srinivas V, Pratyusha S and Gopalakrishnan S, 2021, Streptomyces consortia-mediated plant defense against Fusarium wilt and plant growth-promotion in chickpea. Microbial Pathogenesis, 157: 104961.
Anshu A, Agarwal P, Mishra K, Yadav U, Verma I, Chauhan S, Srivastava P K and Singh P C, 2022, Synergistic action of Trichoderma koningiopsis and T. asperellum mitigates salt stress in paddy. Physiology and Molecular Biology of Plants, 28(5): 987-1004.
Bo T, Kong C, Zou S, Mo M and Liu Y, 2022, Bacillus nematocida B16 enhanced the rhizosphere colonization of Pochonia chlamydosporia ZK7 and controlled the efficacy of the root-knot nematode Meloidogyne incognita. Microorganisms, 10(2): 218.
Bora P, Bora L C, Bhuyan R P, Hashem A and Abd-Allah E F, 2022, Bioagent consortia assisted suppression in grey blight disease with enhanced leaf nutrients and biochemical properties of tea (Camellia sinensis). Biological Control, 170: 104907.
Brakhage A A, 2013, Regulation of fungal secondary metabolism. Nature Reviews Microbiology, 11(1): 21-32.
Chen Z, Xie Y, Qiu S, Li M and Ge S, 2024, Enriched functional exoproteins and increased exopolysaccharides with altered molecular conformation mutually promoted indigenous microalgal-bacterial consortium biofilm growth under high light intensity. Chemical Engineering Journal, 480: 148056.
Cortes-Tolalpa L, Norder J, van Elsas J D and Falcao Salles J, 2018, Halotolerant microbial consortia able to degrade highly recalcitrant plant biomass substrate. Applied microbiology and biotechnology, 102: 2913-2927.
Devi R, Kaur T, Kour D, Yadav A N and Suman A, 2022, Potential applications of mineral solubilizing rhizospheric and nitrogen fixing endophytic bacteria as microbial consortium for the growth promotion of chilli (Capsicum annum L.). Biologia, 77(10): 2933-2943.
Devi N O, Tombisana Devi R K, Debbarma M, Hajong M and Thokchom S, 2022, Effect of endophytic Bacillus and arbuscular mycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. Egyptian Journal of Biological Pest Control, 32: 1-14.
Dukare A S, Prasanna R, Dubey S C, Nain L, Chaudhary V, Singh R and Saxena A K, 2011, Evaluating novel microbe amended composts as biocontrol agents in tomato. Crop protection, 30(4): 436-442.
Gao H, Niu J, Yang H, Lu Z, Zhou L, Meng F, Lu F and Chen M, 2021, Epimerization of deoxynivalenol by the Devosia strain A6-243 assisted by pyrroloquinoline quinone. Toxins, 14(1): 16.
Garbeva P, Hordijk C, Gerards S and De Boer W, 2014, Volatile-mediated interactions between phylogenetically different soil bacteria. Frontiers in microbiology, 5: 289.
Guo Q, Shi M, Chen L, Zhou J, Zhang L, Li Y, Xue Q and Lai H, 2020, The biocontrol agent Streptomyces pactum increases Pseudomonas koreensis populations in the rhizosphere by enhancing chemotaxis and biofilm formation. Soil Biology and Biochemistry, 144: 107755.
Hao D, Lang B, Wang Y, Wang X, Liu T and Chen J, 2022, Designing synthetic consortia of Trichoderma strains that improve antagonistic activities against pathogens and cucumber seedling growth. Microbial Cell Factories, 21(1): 234.
Hegde G M and Vijaykumar K N, 2022, Formulation, Application and Commercialization of Biopesticides in India Formulation, Application and Commercialization of Biopesticides in India. Asia Pacific Biofertilizers and Biopesticides Information Platform.
Huang W, Chang J, Wang P, Liu C, Yin Q, Zhu Q, Lu F and Gao T, 2018, Effect of the combined compound probiotics with mycotoxin degradation enzyme on detoxifying aflatoxin B1 and zearalenone. The Journal of toxicological sciences, 43(6): 377-385.
Jadav P C, Hegde G M, Jahagirdar S and Navi V, 2022, Evaluation of microbial consortia against Sclerotium rolfsii Sacc. causing foot rot of wheat. Journal of Farm Sciences, 35(02): 219-222.
Jain A, Singh A, Singh S and Singh H B, 2015, Phenols enhancement effect of microbial consortium in pea plants restrains Sclerotinia sclerotiorum. Biological Control, 89: 23-32.
Jariyal M, Jindal V, Mandal K, Gupta V K and Singh B, 2018, Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotoxicology and environmental safety, 159: 310-316.
Johnson I, Ramjegathesh R, Sheela J, Shoba N and Maheshwarappa H P, 2017, Development of microbial consortia for the management of leaf blight disease of coconut. Acta Phytopathologica et Entomologica Hungarica, 52(1): 1-14.
Kaur T, Devi R, Kumar S, Sheikh I, Kour D and Yadav A N, 2022, Microbial consortium with nitrogen fixing and mineral solubilizing attributes for growth of barley (Hordeum vulgare L.). Heliyon, 8(4).
Kaur T, Devi R, Kumar S, Kour D and Yadav A N, 2023, Plant growth promotion of pearl millet (Pennisetum glaucum L.) by novel bacterial consortium with multifunctional attributes. Biologia
78(2): 621-631.
Khan B A, Nadeem M A, Nawaz H, Amin M M, Abbasi G H, Nadeem M, Ali M, Ameen M, Javaid M M, Maqbool R and Ikram M, 2023, Pesticides: impacts on agriculture productivity, environment, and management strategies. In Emerging contaminants and plants: Interactions, adaptations and remediation technologies (pp. 109-134). Cham: Springer International Publishing.
Kumar S M, Chowdappa P and Krishna V, 2015, Development of seed coating formulation using consortium of Bacillus subtilis OTPB1 and Trichoderma harzianum OTPB3 for plant growth promotion and induction of systemic resistance in field and horticultural crops. Indian Phytopathology 68(1): 25-31.
Kumar K H and Jagadeesh K S, 2016, Microbial consortia-mediated plant defense against phytopathogens and growth benefits. South Indian Journal of Biological Sciences, 2(4): 395-403.
Kumar S, Chandra R, Behera L, Sudhir I, Meena M, Singh S and Keswani C, 2023, Microbial consortium mediated acceleration of the defense response in potato against Alternaria solani through prodigious inflation in phenylpropanoid derivatives and redox homeostasis. Heliyon, 9(11).
Lashani E, Amoozegar M A, Turner R J. and Moghimi H, 2023, Use of microbial consortia in bioremediation of metalloid polluted environments. Microorganisms, 11(4): 891.
Lutz M P, Wenger S, Maurhofer M, Défago G and Duffy B, 2004, Signaling between bacterial and fungal biocontrol agents in a strain mixture. FEMS microbiology ecology, 48(3): 447-455.
Maciag T, Kozieł E, Rusin P, Otulak-Kozieł K, Jafra S and Czajkowski R, 2023, Microbial consortia for plant protection against diseases: more than the sum of its parts. International Journal of Molecular Sciences, 24(15): 12227.
Maheshwari D K, Dubey R C, Agarwal M, Dheeman S, Aeron A and Bajpai V K, 2015, Carrier based formulations of biocoenotic consortia of disease suppressive Pseudomonas aeruginosa KRP1 and Bacillus licheniformis KRB1. Ecological Engineering, 81: 272-277.
Minchev Z, Kostenko O, Soler R and Pozo M J, 2021, Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Frontiers in Plant Science, 12: 756368.
Mondal A, Mahapatra S, Chakraborty S, Debnath D, Das T and Samanta M, 2024, Eco-friendly Management of Collar Rot of Lentil by Introduced Native Rhizobacterial Candidates. Indian Journal of Agricultural Research, 58(1).
Niu B, Wang W, Yuan Z, Sederoff R R, Sederoff H, Chiang V L and Borriss R, 2020, Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Frontiers in Microbiology, 11: 585404.
Nunes P S, Junior G V L, Mascarin G M, Guimaraes R A, Medeiros F H, Arthurs S and Bettiol W, 2024, Microbial consortium of biological products: do they have a future?. Biological Control,: 105439.
Nurcahyanti S D, Masnilah R, Budiman S A, Tanzil A I and Hendra Nurdika A A, 2024, Microbial consortium formulation in liquid organic fertilizer for managing bacterial leaf blight (Xanthomonas oryzae pv. oryzae), rice blast (Pyricularia oryzae), and enhancing rice productivity. Biodiversitas: Journal of Biological Diversity, 25(5).
Palmieri D, Vitullo D, De Curtis F and Lima G, 2017, A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant and soil, 412: 425-439.
Palmieri D, Ianiri G, Del Grosso C, Barone G, De Curtis F, Castoria R and Lima G, 2022, Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae, 8(7): 577.
Pishchany G, Mevers E, Ndousse-Fetter S, Horvath Jr D J, Paludo C R, Silva-Junior E A, Koren S, Skaar E P, Clardy J and Kolter R, 2018, Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proceedings of the National Academy of Sciences, 115(40): 10124-10129.
Poveda J and Eugui D, 2022, Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biological Control, 176: 105100.
Ram R M, Tripathi R, Birla H, Dilnashin H, Singh S P and Keswani C, 2019, Mixed PGPR consortium: an effective modulator of antioxidant network for management of collar rot in cauliflower. Archives of Phytopathology and Plant Protection, 52(7-8): 844-862.
Resti Z, Liswarni Y and Martinius M, 2020, Endophytic bacterial consortia as biological control of bacterial leaf blight and plant growth promoter of rice (Oryza sativa L.). Journal of Applied Agricultural Science and Technology, 4(2): 134-145.
Ristaino J B, Anderson P K, Bebber D P, Brauman K A, Cunniffe N J, Fedoroff N V, Finegold C, Garrett K A, Gilligan C A, Jones C M and Martin M D, 2021, The persistent threat of emerging plant disease pandemics to global food security. Proceedings of the National Academy of Sciences, 118(23): 2022239118.
Santoyo G, Guzmán-Guzmán P, Parra-Cota F I, Santos-Villalobos S D L, Orozco-Mosqueda, M D C and Glick B R, 2021, Plant growth stimulation by microbial consortia. Agronomy, 11(2): 219.
Sanzani S M, Reverberi M and Geisen R, 2016, Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biology and Technology, 122: 95-105.
Saxena A, Mishra S, Ray S, Raghuwanshi R and Singh H B, 2020, Differential reprogramming of defense network in Capsicum annum L. plants against colletotrichum truncatum infection by phyllospheric and rhizospheric trichoderma strains. Journal of plant growth regulation, 39: 751-763.
Singh A, Sarma B K, Upadhyay R S and Singh H B, 2013, Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiological Research, 168(1): 33-40.
Singh D, Jadon K S, Verma A, Geat N, Sharma R, Meena K K and Kakani R K, 2024, Formulations of synergistic microbial consortia for enhanced systemic resistance against Fusarium wilt in cumin. International Microbiology: 1-27.
Sood D, Sharma A and Sharma M, 2021, Management of bacterial wilt of tomato through consortium of biocontrol agents. Journal of Pharmacognosy and Phytochemistry, 10(1): 1355-1358.
Stockwell V O, Johnson K B, Sugar D and Loper J E, 2011, Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology, 101(1): 113-123.
Taheur F B, Fedhila K, Chaieb K, Kouidhi B, Bakhrouf A and Abrunhosa L, 2017, Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. International journal of food microbiology, 251: 1-7.
Thakkar A and Saraf M, 2015, Development of microbial consortia as a biocontrol agent for effective management of fungal diseases in Glycine max L. Archives of Phytopathology and Plant Protection, 48(6): 459-474.
Thomloudi E E, Tsalgatidou P C, Douka D, Spantidos T N, Dimou M, Venieraki A and Katinakis P, 2019, Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hellenic Plant Protection Journal, 12(2): 61-77.
Trivedi S, Srivastava M, Ratan V, Mishra A, Dixit S and Pandey S, 2020, Evaluation of microbial consortia on systemic resistance against chickpea wilt. Bangladesh Journal of Botany, 49(3): 653-661.
Vespermann A, Kai M and Piechulla B, 2007, Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Applied and environmental microbiology, 73(17): 5639-5641.
Vishwakarma K, Kumar N, Shandilya C, Mohapatra S, Bhayana S and Varma A, 2020, Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Frontiers in Microbiology, 11: 560406.
Walker V, Couillerot O, Von Felten A, Bellvert F, Jansa J, Maurhofer M, Bally R, Moënne-Loccoz Y and Comte G, 2012, Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant and soil, 356: 151-163.
Wang, Yanxia, Gang Wang, Yijun Dai, Yu Wang, Yin-Won Lee, Jianrong Shi, and Jianhong Xu, 2020, "Biodegradation of deoxynivalenol by a novel microbial consortium." Frontiers in microbiology, 10: 2964.
Wang Y, Hu J, Dai Y, Wang Y, Shi J, Wang G, Xu J and De Saeger S, 2023, Design and characterization of an artificial two-strain bacterial consortium for the efficient biodegradation of deoxynivalenol. Biological Control, 179: 105172.
Wang Y, Yang L, Xu J, Xin F and Jiang L, 2023, Applications of synthetic microbial consortia in biological control of mycotoxins and fungi. Current Opinion in Food Science: 101074.
Wong C K F, Saidi N B, Vadamalai G, Teh C Y and Zulperi D, 2019, Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against Fusarium wilt of banana. Journal of applied microbiology, 127(2): 544-555.
Wu D, Wang W, Yao Y, Li H, Wang Q and Niu B, 2023, Microbial interactions within beneficial consortia promote soil health. Science of the Total Environment: 165801.
Xu X, Qin G and Tian S, 2008, Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. International Journal of Food Microbiology, 126(1-2): 153-158.
Xu X M, Jeffries P, Pautasso M and Jeger M J, 2011, Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology, 101(9): 1024-1031.
Zhai Y, Zhong L, Gao H, Lu Z, Bie X, Zhao H, Zhang C and Lu F, 2019, Detoxification of deoxynivalenol by a mixed culture of soil bacteria with 3-epi-deoxynivalenol as the main intermediate. Frontiers in microbiology, 10: 2172.
Zhang J, Ahmed W, Dai Z, Zhou X, He Z, Wei L and Ji G, 2022, Microbial consortia: An engineering tool to suppress clubroot of Chinese cabbage by changing the rhizosphere bacterial community composition. Biology, 11(6): 918.