DOI: https://doi.org/10.56669/YXGK4466
ABSTRACT
Entomopathogenic fungi (EPF) offer a promising solution for sustainable pest management due to their ability to infect and kill insect pests. This review highlights the potential of Purpurecillium takamizusanense isolate TCTeb01, isolated from the lychee stink bug in Taiwan, as a dual-purpose biological agent of bioinsecticide and biofertilizer. TCTeb01 exhibits high pathogenicity against various pests, including the lychee stink bug, melon thrips, and coffee berry borer, while remaining safe for beneficial insects. Its remarkable phosphate solubilization capacity further positions it as a biofertilizer, contributing to enhanced agricultural productivity. Developing TCTeb01 for commercial use necessitates addressing challenges in mass production and formulation to ensure spore viability and compatibility with various application methods. This research underscores the need for regulatory compliance and innovation to support the commercialization of TCTeb01, establishing it as a valuable tool in integrated pest management and sustainable agriculture.
Keywords: entomopathogenic fungus, Purpurecillium takamizusanense, lychee stink bug, biopesticide
INTRODUCTION
Entomopathogenic fungi (EPF) possess a remarkable ability to infect and kill insects. These fungi are widely distributed and can have either restricted or broad host ranges, each exhibiting different biocontrol potentials against arthropod pests. The process by which EPF kill insects begins with the adhesion of spores to the insect's exoskeleton. When environmental conditions, such as temperature and humidity, are suitable, the spores germinate, forming appressoria that exert strong mechanical pressure on the cuticle. This mechanical action, combined with the production of lytic enzymes, leads to the disintegration of the insect’s exoskeleton (Skinner et al., 2014; Lacey et al., 2015). Once the fungal hyphae penetrate the insect’s hemocoel, they begin to grow and proliferate. The destruction of the insect’s body results from both mechanical damage to internal organs caused by the developing hyphae and nutrient depletion (Mascarin and Jaronski, 2016; Fan et al., 2017). This dual mechanism of action highlights the effectiveness of EPF as biological control agents, showcasing their potential to sustainably manage insect populations.
Currently, over 1,000 species of EPF from more than 100 genera are recognized and are being explored for their potential commercialization as biopesticides. These fungi infect a wide range of insect orders, and their use in biocontrol has increased significantly in recent years (Shah et al., 2009). Notably, species belonging to the Ascomycota and Entomophthoromycota phyla are among the most commonly encountered in nature. Within Ascomycota, several genera, such as Metarhizium, Beauveria, Isaria, Ophiocordyceps, Cordyceps, Torubiella, Pochonia, Lecanicillium, and Hirsutella, along with species like Paecilomyces variotii and Purpureocillium lilacinum, have been documented in the literature (Tkaczuk et al., 2015; Jaihan et al., 2016).
Due to their high insecticidal efficacy, these fungi are increasingly used as biopesticides, offering a safer alternative to conventional chemical insecticides. EPF are often regarded as superior to synthetic options because they are safe for humans, environmentally sustainable, and target-specific. Additionally, they tend to be more cost-effective over time, produce fewer residual effects, and help combat resistance issues (Sharma et al., 2023). The application of EPF not only supports organic farming practices but also contributes to more sustainable agricultural methods. Collectively, these advantages underscore the importance of EPF in pest management and their vital role in promoting sustainable agriculture.
Application of Entomopathogenic Fungi in Pest Control
While many EPF have been characterized, not all have been effectively utilized for pest control. The development of biopesticides requires extensive experimental validation. Among the fungi successfully employed in the biopesticide industry, Beauveria bassiana and Metarhizium anisopliae are the most widely used. A total of 171 different types of fungal insecticides are registered worldwide, with Beauveria accounting for 58 types, or 33.9% of the total (Faria et al., 2007). B. bassiana has been effective in controlling over 60 insect species, including locusts, grasshoppers, whiteflies, aphids, armyworms, and termites (Ranjan et al., 2021; Rajula et al., 2021). In Brazil, B. bassiana has successfully managed whiteflies and coffee cherry beetles over large areas (Mascarin et al., 2019). In the U.S., the B. bassiana product ‘Mycotrol’ was registered as early as 1999 for managing forestry and agricultural pests, including grasshoppers, sandflies, thrips, and aphids (Shah and Pell, 2003). As of 2023, there are 14 registered isolates of B. bassiana, primarily in the U.S., Europe, China, and Canada. These products are mainly used to control pests in the orders Hemiptera, Lepidoptera, and Coleoptera (Sharma et al., 2023).
Another common EPF is Metarhizium, which is known for its parasitic ability against over 200 insects, nematodes, and mites across eight different orders. It is widely used to control various agricultural and forestry pests, including locusts, cockroaches, termites, rice planthoppers, and Spodoptera litura (St. Leger and Wang, 2010). M. anisopliae has been employed as a biological control agent for many years, particularly in Brazil, where it has demonstrated effectiveness against spittlebugs in sugarcane (Li et al., 2010). Additionally, M. acridum has been extensively produced for locust control. Notably, Green Muscle®, developed by CABI Bioscience, has been successfully registered and implemented in Africa, where it is widely used to control desert locusts (Schistocerca gregaria) (Peng et al., 2008).
The success of these fungi in pest control highlights their potential as environmentally friendly alternatives to chemical pesticides, reinforcing their role in sustainable agricultural practices.
Native entomopathogenic fungus in Taiwan: Purpurecillium takamizusanense isolate TCTeb01
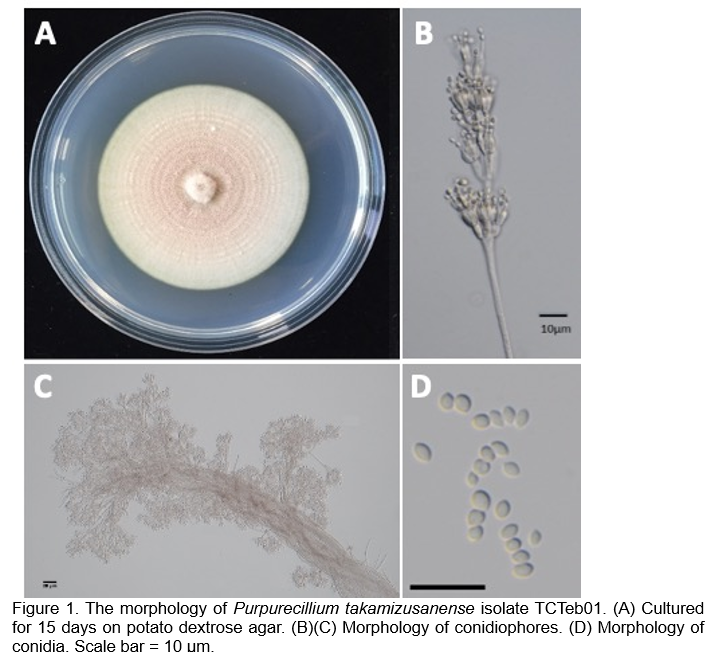
Purpurecillium takamizusanense isolate TCTeb01 is a recently discovered EPF with significant potential for biological pest control. It was isolated from the lychee stink bug (Tessaratoma papillosa Drury) in a logan orchard in Taiwan in 2018. It was identified based on its morphological and molecular biological characteristics, including sequences of the internal transcribed spacer (ITS) and translation elongation factor (TEF) (Lo et al., 2019). Notably, TCTeb01 can produce large quantities of pale purple conidia, which serve as the infectious elements (Figure 1).
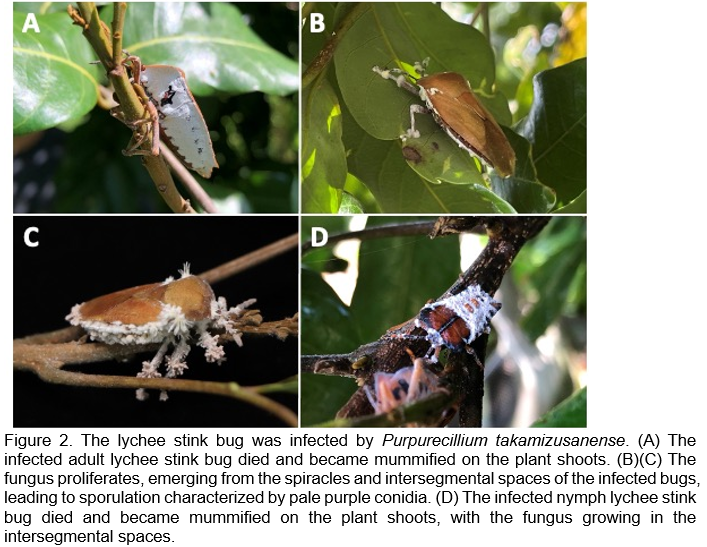
This fungus can infect both the adult and nymph stages of the lychee stink bug, causing infected individuals to die and become mummified on plant shoots. Under high humidity conditions, the fungus proliferates and emerges from the spiracles and interspaces of the bugs, leading to sporulation (Figure 2). The conidia can then be dispersed by wind or rain, facilitating further infections in other bugs.
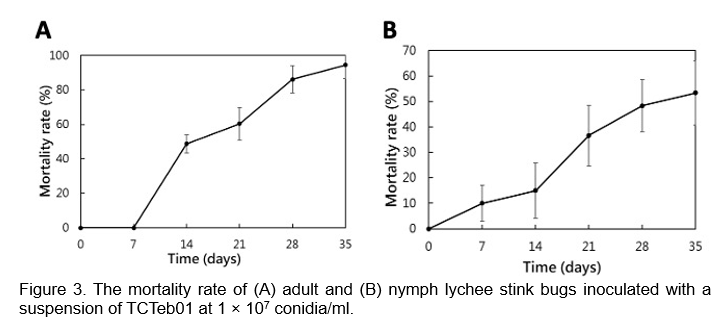
Infectious tests in the greenhouse indicated that when lychee stink bugs were inoculated with a suspension of 1 × 10⁷ conidia/ml, adult mortality began at 14 days post-inoculation and reached 86.1% at 28 days. In contrast, nymphs showed a mortality rate of approximately 48.3% after 28 days under greenhouse conditions (Figure 3). In our preliminary field trial, the wettable powder of TCTeb01 showed 86% control rate. These findings demonstrate the high efficacy of TCTeb01 against lychee stink bugs, underscoring its potential as a bioinsecticide.
While P. takamizusanense has been previously reported in Japan, this is the first documented case of its infection of lychee stink bugs in Taiwan. It is closely related to P. lilacinum, which is commonly isolated from soil and has proven effective against various pests, including nematodes, thrips, bugs, beetles, aphids, and whiteflies (Goffré and Folgarait, 2015). Given these findings, we aimed to explore the potential of P. takamizusanense TCTeb01 for controlling other pests within the same genus.
 \
\
Evaluation of the capacity of Purpurecillium takamizusanense isolate TCTeb01 developed as a biopesticide
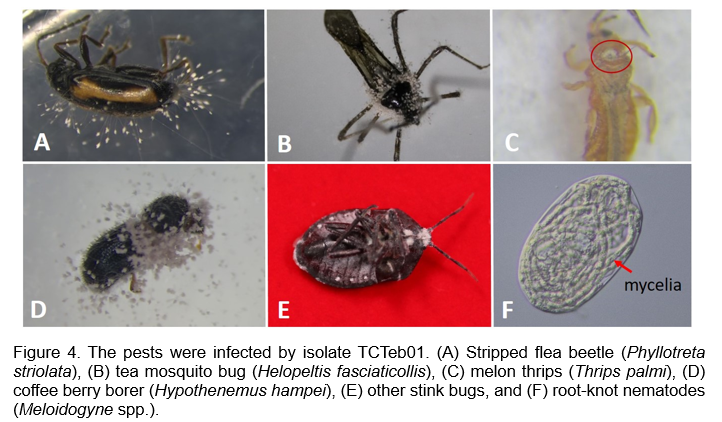
The investigation into TCTeb01's effectiveness against lychee stink bugs highlights its potential as a bio-insecticide, but further considerations regarding mass production, formulation, host range, and safety for non-target organisms are essential for its field application. Tests assessing the host range of TCTeb01 revealed its capacity to infect a variety of pests, including the stripped flea beetle (Phyllotreta striolata), turnip aphid (Lipaphis erysimi), melon thrips (Thrips palmi), oriental fruit fly (Bactrocera dorsalis), tea mosquito bug (Helopeltis fasciaticollis), coffee berry borer (Hypothenemus hampei), and other stink bugs. Notably, TCTeb01 exhibited high efficacy against melon thrips, achieving a mortality rate of 77.5% by seven days post-inoculation. Furthermore, in adult coffee berry borers, the mortality rate reached an impressive 100% within six days. These pests are notoriously difficult to control with conventional chemical pesticides in Taiwan, underscoring TCTeb01's potential as a key component of integrated pest management (IPM) strategies (Figure 4 A to E).
In addition to its effectiveness against certain pests, TCTeb01 has also been shown to infect the eggs of root-knot nematodes, with the eggs being colonized by the mycelia of TCTeb01, which significantly inhibits their hatching (Figure 4 F). Additionally, TCTeb01 demonstrated the ability to survive in soil for over a year, indicating its potential for soil application in controlling root-knot nematodes.
As TCTeb01 is intended for use as a bio-insecticide in the field, evaluating its safety for beneficial insects is crucial. Our studies confirmed that TCTeb01 is safe for honeybees (Apis mellifera) as well as for other beneficial insects such as Eocanthecona furcellata and Mallada basalis. TCTeb01 exhibited high specificity towards targeted pests and demonstrated no adverse effects on the environment.
Overall, TCTeb01 shows significant promise as a sustainable alternative in pest management strategies while aligning with ecological safety standards.
Evaluation of the capacity of Purpurecillium takamizusanense isolate TCTeb01 developed as a biofertilizer
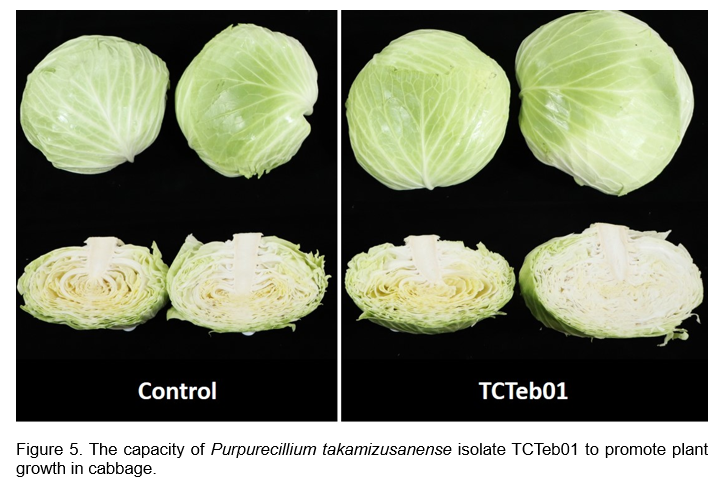
Purpurecillium sp. has been reported to possess significant potential as a biofertilizer. In this context, the plant growth-promoting capabilities of P. takamizusanense TCTeb01 have been demonstrated through its remarkable phosphate solubilization activity, recorded at an impressive rate of 4,672.1 μg mL⁻¹ day⁻¹. To further assess its effectiveness, TCTeb01 was tested for plant growth promotion in various vegetable crops. In cabbage, after treating the plants with the spore suspension of TCTeb01 weekly during the growth period, the plant weight increased by 20% compared to the control group in the field (Figure 5). Specifically, it resulted in a significant 15% increase in yield for water spinach compared to the control group in the field. These findings highlight the potential of TCTeb01 as a viable biofertilizer, offering a sustainable approach to enhancing agricultural productivity.
In addition to its phosphate solubilization capabilities, TCTeb01 may contribute to improved nutrient uptake, enhanced root development, and increased resistance to environmental stressors in plants. By incorporating TCTeb01 into agricultural practices, farmers could not only improve crop yields but also promote soil health and reduce dependency on chemical fertilizers, aligning with the principles of sustainable agriculture.
Overall, the promising results from TCTeb01 underscore its potential role in modern agriculture as an eco-friendly alternative to conventional fertilizers, paving the way for more resilient and productive farming systems.
The way to develop Purpurecillium takamizusanense isolateTCTeb01 as the commercial bioinsecticide in Taiwan
The development of the EPF Purpurecillium takamizusanense isolate TCTeb01 as a bioinsecticide involves several key steps. Initially, selecting a high-potential isolate based on pathogenicity tests is essential. In this regard, TCTeb01 has demonstrated significant effectiveness in controlling lychee stink bugs. The next step is to establish the efficacy of TCTeb01 against these pests.
For successful commercialization, a critical aspect is the establishment of mass production techniques for the EPF. Established EPFs, such as Beauveria, Metarhizium, and Isaria, are readily available on a commercial scale due to their ease of mass production.
Two primary fermentation methods are utilized for mass production: liquid fermentation and solid fermentation. In liquid fermentation, the fungus typically produces blastospores. While Beauveria, Metarhizium, and Isaria can grow as blastospores under appropriate conditions, these are generally more environmentally fragile than aerial conidia. Solid fermentation is the more common method for producing mass conidia, which are the most effective propagules for EPF in natural environments. Most entomopathogenic hyphomycete fungi generate large quantities of small, hydrophobic conidia in thick masses (Wraight, Jackson, and de Kock, 2001). These conidia serve as the primary inocula for EPF to attach to and infect insects.
To produce mass conidia from TCTeb01, we cultured the fungus on rice as a solid substrate. After 14 days of cultivation, we successfully generated over 10¹⁰ conidia per gram of rice substrate. We developed a method to collect these conidia and produce a conidia powder, which can be used as a technical concentrate for formulating water-dispersible powders or granules (Figure 6).
An important consideration for the bioinsecticide formulation is its shelf life. The conidia powder produced from our process can be stored at room temperature (28°C) for at least six months without significant loss of viability. Traditional powdered formulations of fungi, such as B. bassiana and Isaria fumosorosea, have posed challenges in handling, particularly during the wetting process before field application. However, recent research has shown that both vegetable- and petroleum-derived oils can be compatible with these fungi, facilitating easier handling. Similarly, we plan to formulate TCTeb01 conidia powder into either a wettable powder or a wettable dispersal oil to enhance application efficacy.
In Taiwan, developing EPF as bioinsecticides requires compliance with Good Laboratory Practice (GLP) standards, as well as physicochemical properties and toxicological testing. An Experimental Use Permit (EUP) for field trials is also essential for regulatory registration. Additionally, key factors to consider for commercial production include ecological interactions, economic viability, stability, and efficiency (Humber, 2008). The main components of EPF-based biopesticides typically include active ingredients (conidia or dry spores), surfactants, carriers, UV protectants, and stickers. The overall quality of the final product largely depends on the viability of the conidia (Islam et al., 2021). By focusing on these critical aspects, TCTeb01 has the potential to become a valuable addition to sustainable pest management practices.

The challenges of developing TCTeb01 as a bioinsecticide in Taiwan
In Taiwan, only one commercial bioinsecticide product has been registered since 2018, primarily featuring B. bassiana as its active ingredient for controlling the diamondback moth (Plutella xylostella). This product is cultivated on a solid substrate and requires low-temperature storage to maintain its efficacy. Prior to application, it must be treated with a surfactant to create a spore suspension.
Globally, various formulations of EPF, such as wettable powders and dispersal oils, exist. However, no EPF products in these formulations have been registered in Taiwan to date. This situation presents several challenges for the commercialization of EPF in the region, particularly regarding the need for efficient mass production and effective storage solutions.
The primary challenge we face is producing large quantities of conidia and effectively collecting them from solid substrates for subsequent use. Maintaining spore viability at room temperature poses a significant hurdle. To address this, we have developed a mass production process using solid fermentation on rice, yielding over 10¹⁰ conidia per gram. Additionally, we have created a conidia powder that can be stored at room temperature for at least six months, thus overcoming some of the limitations faced by EPFs in Taiwan.
Currently, we are exploring ways to enhance the hydrophilicity of the conidia powder by blending it with other formulations, ensuring better compatibility with water and preserving spore activity. Our goal is also to extend the shelf life of the final product. Future research should focus on maintaining a high level of effectiveness in stored formulations, addressing previous reports of low infection rates (Wraight et al., 2001). By tackling these challenges, we hope to contribute to the successful commercialization of EPF in Taiwan, paving the way for more sustainable pest management solutions.
CONCLUSION
The exploration of P. takamizusanense TCTeb01 reveals its significant dual potential as a bioinsecticide and biofertilizer, aligning with sustainable agricultural practices. Addressing challenges in mass production and formulation is crucial for its commercialization in Taiwan. By enhancing storage methods and application techniques, TCTeb01 can be positioned as a vital component in IPM, contributing to eco-friendly farming and sustainable food production systems.
REFERENCES
de Faria, M. R., and Wraight, S. P. 2007. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biological Control, 43(3): 237-256.
Dannon, H. F., Dannon, A. E., Douro-Kpindou, O. K., Zinsou, A. V., Houndete, A. T., Toffa-Mehinto, J., Elegbede, I.A.T.M., Olou, B.D., Tamo, M. 2020. Toward the efficient use of Beauveria bassiana in integrated cotton insect pest management. Journal of Cotton Research, 3: 1-21.
Fan, Y., Liu, X., Keyhani, N. O., Tang, G., Pei, Y., Zhang, W., and Tong, S. 2017. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proceedings of the National Academy of Sciences, 114(9): E1578-E1586.
Goffré, D. and Folgarait, P. J. 2015. Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. Journal of invertebrate pathology, 130:107-115.
Humber, R. A. 2008. Evolution of entomopathogenicity in fungi. Journal of Invertebrate Pathology, 98(3): 262-266.
Islam, W., Adnan, M., Shabbir, A., Naveed, H., Abubakar, Y. S., Qasim, M., Tayyab, M, Noman, A., Nisar, M. S., Khan, K. A., Ali, H. 2021. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microbial Pathogenesis, 159: 105122.
Jaihan, P., Sangdee, K., and Sangdee, A. 2016. Selection of entomopathogenic fungus for biological control of chili anthracnose disease caused by Colletotrichum spp. European Journal of Plant Pathology, 146: 551-564.
Khan, S., Guo, L., Maimaiti, Y., Mijit, M., and Qiu, D. 2012. Entomopathogenic fungi as microbial biocontrol agent. Molecular Plant Breeding, 3(7).
Lacey, L. A., Grzywacz, D., Shapiro-Ilan, D. I., Frutos, R., Brownbridge, M., and Goettel, M. S. 2015. Insect pathogens as biological control agents: Back to the future. Journal of Invertebrate Pathology, 132: 1-41.
Li, Z., Alves, S. B., Roberts, D. W., Fan, M., Delalibera I. Jr., Tang J. 2010. Biological control of insects in Brazil and China: history, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Science and Technology, 20(2): 117-136.
Lo, P. H., Yu, Y. C., 2019. First report of Purpureocillium takamizusanense as an entomopathogenic fungus infecting Tessaratoma papillosa (Drury) in Taiwan. Journal of Plant Medicine, 61(2&3): 27-30.
Mascarin, G. M., Lopes, R. B., Delalibera Jr, Í., Fernandes, É. K. K., Luz, C., and Faria, M. 2019. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. Journal of Invertebrate Pathology, 165: 46-53.
Mascarin, G. M., and Jaronski, S. T. 2016. The production and uses of Beauveria bassiana as a microbial insecticide. World Journal of Microbiology and Biotechnology, 32: 1-26.
Peng, G., Wang, Z., Yin, Y., Zeng, D., and Xia, Y. 2008. Field trials of Metarhizium anisopliae var. acridum (Ascomycota: Hypocreales) against oriental migratory locusts, Locusta migratoria manilensis (Meyen) in Northern China. Crop Protection, 27(9): 1244-1250.
Ranjan, V., Sharma, M., and Sur, A. 2021. Beauveria bassiana as a potent biopesticide for control of locust: A review. Research Reports, 5.
Rajula, J., Karthi, S., Mumba, S., Pittarate, S., Thungrabeab, M., and Krutmuang, P. 2021. Current status and future prospects of entomopathogenic fungi: A potential source of biopesticides. Recent advancement in microbial biotechnology, 71-98.
Shah, F. A., Ansari, M. A., Watkins, J., Phelps, Z., Cross, J., and Butt, T. M. 2009. Biocontrol science and technology influence of commercial fungicides on the germination, growth, and virulence of four species of entomopathogenic fungi. Biocontrol Science and Technology, 19(7): 743-753.
Shah, P. A., and Pell, J. K. 2003. Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology, 61(5): 413-423.
Sharma, A., Sharma, S., and Yadav, P. K. 2023. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent Food & Agriculture, 9(1): 2180857.
Shah, P. A. and Pell, J. K. 2003. Entomopathogenic fungi as biological control agents. Applied microbiology and biotechnology, 61(5): 413-423.
Skinner M., Parker B. L., Kim J. S. 2014. Role of entomopathogenic fungi. In: Abrol DP (ed) Integrated pest management. Academic Press, Cambridge, pp 169-191.
St. Leger, R. J., and Wang, C. 2010. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Applied Microbiology and Biotechnology, 85: 901-907.
Tkaczuk, C., Harasimiuk, M., Król, A., and Bereś, P. K. 2015. The effect of selected pesticides on the growth of entomopathogenic fungi Hirsutella nodulosa and Beauveria bassiana. Journal of Ecological Engineering, 16(3):177-183
Wraight, S. P., Jackson, M. A., and de Kock, S. L. 2001. Production, stabilization and formulation of fungal biocontrol agents. In T. M. Butt, C. W. Jackson, and N. Magan (Eds.), Fungi as biocontrol agents: Progress, problems and potential. Wallingford, UK: CABI Publishing.
Advancing the Development of Entomopathogenic Fungus Purpureocillium takamizusanense TCTeb01: Challenges and Opportunities in Taiwan
DOI: https://doi.org/10.56669/YXGK4466
ABSTRACT
Entomopathogenic fungi (EPF) offer a promising solution for sustainable pest management due to their ability to infect and kill insect pests. This review highlights the potential of Purpurecillium takamizusanense isolate TCTeb01, isolated from the lychee stink bug in Taiwan, as a dual-purpose biological agent of bioinsecticide and biofertilizer. TCTeb01 exhibits high pathogenicity against various pests, including the lychee stink bug, melon thrips, and coffee berry borer, while remaining safe for beneficial insects. Its remarkable phosphate solubilization capacity further positions it as a biofertilizer, contributing to enhanced agricultural productivity. Developing TCTeb01 for commercial use necessitates addressing challenges in mass production and formulation to ensure spore viability and compatibility with various application methods. This research underscores the need for regulatory compliance and innovation to support the commercialization of TCTeb01, establishing it as a valuable tool in integrated pest management and sustainable agriculture.
Keywords: entomopathogenic fungus, Purpurecillium takamizusanense, lychee stink bug, biopesticide
INTRODUCTION
Entomopathogenic fungi (EPF) possess a remarkable ability to infect and kill insects. These fungi are widely distributed and can have either restricted or broad host ranges, each exhibiting different biocontrol potentials against arthropod pests. The process by which EPF kill insects begins with the adhesion of spores to the insect's exoskeleton. When environmental conditions, such as temperature and humidity, are suitable, the spores germinate, forming appressoria that exert strong mechanical pressure on the cuticle. This mechanical action, combined with the production of lytic enzymes, leads to the disintegration of the insect’s exoskeleton (Skinner et al., 2014; Lacey et al., 2015). Once the fungal hyphae penetrate the insect’s hemocoel, they begin to grow and proliferate. The destruction of the insect’s body results from both mechanical damage to internal organs caused by the developing hyphae and nutrient depletion (Mascarin and Jaronski, 2016; Fan et al., 2017). This dual mechanism of action highlights the effectiveness of EPF as biological control agents, showcasing their potential to sustainably manage insect populations.
Currently, over 1,000 species of EPF from more than 100 genera are recognized and are being explored for their potential commercialization as biopesticides. These fungi infect a wide range of insect orders, and their use in biocontrol has increased significantly in recent years (Shah et al., 2009). Notably, species belonging to the Ascomycota and Entomophthoromycota phyla are among the most commonly encountered in nature. Within Ascomycota, several genera, such as Metarhizium, Beauveria, Isaria, Ophiocordyceps, Cordyceps, Torubiella, Pochonia, Lecanicillium, and Hirsutella, along with species like Paecilomyces variotii and Purpureocillium lilacinum, have been documented in the literature (Tkaczuk et al., 2015; Jaihan et al., 2016).
Due to their high insecticidal efficacy, these fungi are increasingly used as biopesticides, offering a safer alternative to conventional chemical insecticides. EPF are often regarded as superior to synthetic options because they are safe for humans, environmentally sustainable, and target-specific. Additionally, they tend to be more cost-effective over time, produce fewer residual effects, and help combat resistance issues (Sharma et al., 2023). The application of EPF not only supports organic farming practices but also contributes to more sustainable agricultural methods. Collectively, these advantages underscore the importance of EPF in pest management and their vital role in promoting sustainable agriculture.
Application of Entomopathogenic Fungi in Pest Control
While many EPF have been characterized, not all have been effectively utilized for pest control. The development of biopesticides requires extensive experimental validation. Among the fungi successfully employed in the biopesticide industry, Beauveria bassiana and Metarhizium anisopliae are the most widely used. A total of 171 different types of fungal insecticides are registered worldwide, with Beauveria accounting for 58 types, or 33.9% of the total (Faria et al., 2007). B. bassiana has been effective in controlling over 60 insect species, including locusts, grasshoppers, whiteflies, aphids, armyworms, and termites (Ranjan et al., 2021; Rajula et al., 2021). In Brazil, B. bassiana has successfully managed whiteflies and coffee cherry beetles over large areas (Mascarin et al., 2019). In the U.S., the B. bassiana product ‘Mycotrol’ was registered as early as 1999 for managing forestry and agricultural pests, including grasshoppers, sandflies, thrips, and aphids (Shah and Pell, 2003). As of 2023, there are 14 registered isolates of B. bassiana, primarily in the U.S., Europe, China, and Canada. These products are mainly used to control pests in the orders Hemiptera, Lepidoptera, and Coleoptera (Sharma et al., 2023).
Another common EPF is Metarhizium, which is known for its parasitic ability against over 200 insects, nematodes, and mites across eight different orders. It is widely used to control various agricultural and forestry pests, including locusts, cockroaches, termites, rice planthoppers, and Spodoptera litura (St. Leger and Wang, 2010). M. anisopliae has been employed as a biological control agent for many years, particularly in Brazil, where it has demonstrated effectiveness against spittlebugs in sugarcane (Li et al., 2010). Additionally, M. acridum has been extensively produced for locust control. Notably, Green Muscle®, developed by CABI Bioscience, has been successfully registered and implemented in Africa, where it is widely used to control desert locusts (Schistocerca gregaria) (Peng et al., 2008).
The success of these fungi in pest control highlights their potential as environmentally friendly alternatives to chemical pesticides, reinforcing their role in sustainable agricultural practices.
Native entomopathogenic fungus in Taiwan: Purpurecillium takamizusanense isolate TCTeb01
Purpurecillium takamizusanense isolate TCTeb01 is a recently discovered EPF with significant potential for biological pest control. It was isolated from the lychee stink bug (Tessaratoma papillosa Drury) in a logan orchard in Taiwan in 2018. It was identified based on its morphological and molecular biological characteristics, including sequences of the internal transcribed spacer (ITS) and translation elongation factor (TEF) (Lo et al., 2019). Notably, TCTeb01 can produce large quantities of pale purple conidia, which serve as the infectious elements (Figure 1).
This fungus can infect both the adult and nymph stages of the lychee stink bug, causing infected individuals to die and become mummified on plant shoots. Under high humidity conditions, the fungus proliferates and emerges from the spiracles and interspaces of the bugs, leading to sporulation (Figure 2). The conidia can then be dispersed by wind or rain, facilitating further infections in other bugs.
Infectious tests in the greenhouse indicated that when lychee stink bugs were inoculated with a suspension of 1 × 10⁷ conidia/ml, adult mortality began at 14 days post-inoculation and reached 86.1% at 28 days. In contrast, nymphs showed a mortality rate of approximately 48.3% after 28 days under greenhouse conditions (Figure 3). In our preliminary field trial, the wettable powder of TCTeb01 showed 86% control rate. These findings demonstrate the high efficacy of TCTeb01 against lychee stink bugs, underscoring its potential as a bioinsecticide.
While P. takamizusanense has been previously reported in Japan, this is the first documented case of its infection of lychee stink bugs in Taiwan. It is closely related to P. lilacinum, which is commonly isolated from soil and has proven effective against various pests, including nematodes, thrips, bugs, beetles, aphids, and whiteflies (Goffré and Folgarait, 2015). Given these findings, we aimed to explore the potential of P. takamizusanense TCTeb01 for controlling other pests within the same genus.
Evaluation of the capacity of Purpurecillium takamizusanense isolate TCTeb01 developed as a biopesticide
The investigation into TCTeb01's effectiveness against lychee stink bugs highlights its potential as a bio-insecticide, but further considerations regarding mass production, formulation, host range, and safety for non-target organisms are essential for its field application. Tests assessing the host range of TCTeb01 revealed its capacity to infect a variety of pests, including the stripped flea beetle (Phyllotreta striolata), turnip aphid (Lipaphis erysimi), melon thrips (Thrips palmi), oriental fruit fly (Bactrocera dorsalis), tea mosquito bug (Helopeltis fasciaticollis), coffee berry borer (Hypothenemus hampei), and other stink bugs. Notably, TCTeb01 exhibited high efficacy against melon thrips, achieving a mortality rate of 77.5% by seven days post-inoculation. Furthermore, in adult coffee berry borers, the mortality rate reached an impressive 100% within six days. These pests are notoriously difficult to control with conventional chemical pesticides in Taiwan, underscoring TCTeb01's potential as a key component of integrated pest management (IPM) strategies (Figure 4 A to E).
In addition to its effectiveness against certain pests, TCTeb01 has also been shown to infect the eggs of root-knot nematodes, with the eggs being colonized by the mycelia of TCTeb01, which significantly inhibits their hatching (Figure 4 F). Additionally, TCTeb01 demonstrated the ability to survive in soil for over a year, indicating its potential for soil application in controlling root-knot nematodes.
As TCTeb01 is intended for use as a bio-insecticide in the field, evaluating its safety for beneficial insects is crucial. Our studies confirmed that TCTeb01 is safe for honeybees (Apis mellifera) as well as for other beneficial insects such as Eocanthecona furcellata and Mallada basalis. TCTeb01 exhibited high specificity towards targeted pests and demonstrated no adverse effects on the environment.
Overall, TCTeb01 shows significant promise as a sustainable alternative in pest management strategies while aligning with ecological safety standards.
Evaluation of the capacity of Purpurecillium takamizusanense isolate TCTeb01 developed as a biofertilizer
Purpurecillium sp. has been reported to possess significant potential as a biofertilizer. In this context, the plant growth-promoting capabilities of P. takamizusanense TCTeb01 have been demonstrated through its remarkable phosphate solubilization activity, recorded at an impressive rate of 4,672.1 μg mL⁻¹ day⁻¹. To further assess its effectiveness, TCTeb01 was tested for plant growth promotion in various vegetable crops. In cabbage, after treating the plants with the spore suspension of TCTeb01 weekly during the growth period, the plant weight increased by 20% compared to the control group in the field (Figure 5). Specifically, it resulted in a significant 15% increase in yield for water spinach compared to the control group in the field. These findings highlight the potential of TCTeb01 as a viable biofertilizer, offering a sustainable approach to enhancing agricultural productivity.
In addition to its phosphate solubilization capabilities, TCTeb01 may contribute to improved nutrient uptake, enhanced root development, and increased resistance to environmental stressors in plants. By incorporating TCTeb01 into agricultural practices, farmers could not only improve crop yields but also promote soil health and reduce dependency on chemical fertilizers, aligning with the principles of sustainable agriculture.
Overall, the promising results from TCTeb01 underscore its potential role in modern agriculture as an eco-friendly alternative to conventional fertilizers, paving the way for more resilient and productive farming systems.
The way to develop Purpurecillium takamizusanense isolateTCTeb01 as the commercial bioinsecticide in Taiwan
The development of the EPF Purpurecillium takamizusanense isolate TCTeb01 as a bioinsecticide involves several key steps. Initially, selecting a high-potential isolate based on pathogenicity tests is essential. In this regard, TCTeb01 has demonstrated significant effectiveness in controlling lychee stink bugs. The next step is to establish the efficacy of TCTeb01 against these pests.
For successful commercialization, a critical aspect is the establishment of mass production techniques for the EPF. Established EPFs, such as Beauveria, Metarhizium, and Isaria, are readily available on a commercial scale due to their ease of mass production.
Two primary fermentation methods are utilized for mass production: liquid fermentation and solid fermentation. In liquid fermentation, the fungus typically produces blastospores. While Beauveria, Metarhizium, and Isaria can grow as blastospores under appropriate conditions, these are generally more environmentally fragile than aerial conidia. Solid fermentation is the more common method for producing mass conidia, which are the most effective propagules for EPF in natural environments. Most entomopathogenic hyphomycete fungi generate large quantities of small, hydrophobic conidia in thick masses (Wraight, Jackson, and de Kock, 2001). These conidia serve as the primary inocula for EPF to attach to and infect insects.
To produce mass conidia from TCTeb01, we cultured the fungus on rice as a solid substrate. After 14 days of cultivation, we successfully generated over 10¹⁰ conidia per gram of rice substrate. We developed a method to collect these conidia and produce a conidia powder, which can be used as a technical concentrate for formulating water-dispersible powders or granules (Figure 6).
An important consideration for the bioinsecticide formulation is its shelf life. The conidia powder produced from our process can be stored at room temperature (28°C) for at least six months without significant loss of viability. Traditional powdered formulations of fungi, such as B. bassiana and Isaria fumosorosea, have posed challenges in handling, particularly during the wetting process before field application. However, recent research has shown that both vegetable- and petroleum-derived oils can be compatible with these fungi, facilitating easier handling. Similarly, we plan to formulate TCTeb01 conidia powder into either a wettable powder or a wettable dispersal oil to enhance application efficacy.
In Taiwan, developing EPF as bioinsecticides requires compliance with Good Laboratory Practice (GLP) standards, as well as physicochemical properties and toxicological testing. An Experimental Use Permit (EUP) for field trials is also essential for regulatory registration. Additionally, key factors to consider for commercial production include ecological interactions, economic viability, stability, and efficiency (Humber, 2008). The main components of EPF-based biopesticides typically include active ingredients (conidia or dry spores), surfactants, carriers, UV protectants, and stickers. The overall quality of the final product largely depends on the viability of the conidia (Islam et al., 2021). By focusing on these critical aspects, TCTeb01 has the potential to become a valuable addition to sustainable pest management practices.
The challenges of developing TCTeb01 as a bioinsecticide in Taiwan
In Taiwan, only one commercial bioinsecticide product has been registered since 2018, primarily featuring B. bassiana as its active ingredient for controlling the diamondback moth (Plutella xylostella). This product is cultivated on a solid substrate and requires low-temperature storage to maintain its efficacy. Prior to application, it must be treated with a surfactant to create a spore suspension.
Globally, various formulations of EPF, such as wettable powders and dispersal oils, exist. However, no EPF products in these formulations have been registered in Taiwan to date. This situation presents several challenges for the commercialization of EPF in the region, particularly regarding the need for efficient mass production and effective storage solutions.
The primary challenge we face is producing large quantities of conidia and effectively collecting them from solid substrates for subsequent use. Maintaining spore viability at room temperature poses a significant hurdle. To address this, we have developed a mass production process using solid fermentation on rice, yielding over 10¹⁰ conidia per gram. Additionally, we have created a conidia powder that can be stored at room temperature for at least six months, thus overcoming some of the limitations faced by EPFs in Taiwan.
Currently, we are exploring ways to enhance the hydrophilicity of the conidia powder by blending it with other formulations, ensuring better compatibility with water and preserving spore activity. Our goal is also to extend the shelf life of the final product. Future research should focus on maintaining a high level of effectiveness in stored formulations, addressing previous reports of low infection rates (Wraight et al., 2001). By tackling these challenges, we hope to contribute to the successful commercialization of EPF in Taiwan, paving the way for more sustainable pest management solutions.
CONCLUSION
The exploration of P. takamizusanense TCTeb01 reveals its significant dual potential as a bioinsecticide and biofertilizer, aligning with sustainable agricultural practices. Addressing challenges in mass production and formulation is crucial for its commercialization in Taiwan. By enhancing storage methods and application techniques, TCTeb01 can be positioned as a vital component in IPM, contributing to eco-friendly farming and sustainable food production systems.
REFERENCES
de Faria, M. R., and Wraight, S. P. 2007. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biological Control, 43(3): 237-256.
Dannon, H. F., Dannon, A. E., Douro-Kpindou, O. K., Zinsou, A. V., Houndete, A. T., Toffa-Mehinto, J., Elegbede, I.A.T.M., Olou, B.D., Tamo, M. 2020. Toward the efficient use of Beauveria bassiana in integrated cotton insect pest management. Journal of Cotton Research, 3: 1-21.
Fan, Y., Liu, X., Keyhani, N. O., Tang, G., Pei, Y., Zhang, W., and Tong, S. 2017. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proceedings of the National Academy of Sciences, 114(9): E1578-E1586.
Goffré, D. and Folgarait, P. J. 2015. Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. Journal of invertebrate pathology, 130:107-115.
Humber, R. A. 2008. Evolution of entomopathogenicity in fungi. Journal of Invertebrate Pathology, 98(3): 262-266.
Islam, W., Adnan, M., Shabbir, A., Naveed, H., Abubakar, Y. S., Qasim, M., Tayyab, M, Noman, A., Nisar, M. S., Khan, K. A., Ali, H. 2021. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microbial Pathogenesis, 159: 105122.
Jaihan, P., Sangdee, K., and Sangdee, A. 2016. Selection of entomopathogenic fungus for biological control of chili anthracnose disease caused by Colletotrichum spp. European Journal of Plant Pathology, 146: 551-564.
Khan, S., Guo, L., Maimaiti, Y., Mijit, M., and Qiu, D. 2012. Entomopathogenic fungi as microbial biocontrol agent. Molecular Plant Breeding, 3(7).
Lacey, L. A., Grzywacz, D., Shapiro-Ilan, D. I., Frutos, R., Brownbridge, M., and Goettel, M. S. 2015. Insect pathogens as biological control agents: Back to the future. Journal of Invertebrate Pathology, 132: 1-41.
Li, Z., Alves, S. B., Roberts, D. W., Fan, M., Delalibera I. Jr., Tang J. 2010. Biological control of insects in Brazil and China: history, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Science and Technology, 20(2): 117-136.
Lo, P. H., Yu, Y. C., 2019. First report of Purpureocillium takamizusanense as an entomopathogenic fungus infecting Tessaratoma papillosa (Drury) in Taiwan. Journal of Plant Medicine, 61(2&3): 27-30.
Mascarin, G. M., Lopes, R. B., Delalibera Jr, Í., Fernandes, É. K. K., Luz, C., and Faria, M. 2019. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. Journal of Invertebrate Pathology, 165: 46-53.
Mascarin, G. M., and Jaronski, S. T. 2016. The production and uses of Beauveria bassiana as a microbial insecticide. World Journal of Microbiology and Biotechnology, 32: 1-26.
Peng, G., Wang, Z., Yin, Y., Zeng, D., and Xia, Y. 2008. Field trials of Metarhizium anisopliae var. acridum (Ascomycota: Hypocreales) against oriental migratory locusts, Locusta migratoria manilensis (Meyen) in Northern China. Crop Protection, 27(9): 1244-1250.
Ranjan, V., Sharma, M., and Sur, A. 2021. Beauveria bassiana as a potent biopesticide for control of locust: A review. Research Reports, 5.
Rajula, J., Karthi, S., Mumba, S., Pittarate, S., Thungrabeab, M., and Krutmuang, P. 2021. Current status and future prospects of entomopathogenic fungi: A potential source of biopesticides. Recent advancement in microbial biotechnology, 71-98.
Shah, F. A., Ansari, M. A., Watkins, J., Phelps, Z., Cross, J., and Butt, T. M. 2009. Biocontrol science and technology influence of commercial fungicides on the germination, growth, and virulence of four species of entomopathogenic fungi. Biocontrol Science and Technology, 19(7): 743-753.
Shah, P. A., and Pell, J. K. 2003. Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology, 61(5): 413-423.
Sharma, A., Sharma, S., and Yadav, P. K. 2023. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent Food & Agriculture, 9(1): 2180857.
Shah, P. A. and Pell, J. K. 2003. Entomopathogenic fungi as biological control agents. Applied microbiology and biotechnology, 61(5): 413-423.
Skinner M., Parker B. L., Kim J. S. 2014. Role of entomopathogenic fungi. In: Abrol DP (ed) Integrated pest management. Academic Press, Cambridge, pp 169-191.
St. Leger, R. J., and Wang, C. 2010. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Applied Microbiology and Biotechnology, 85: 901-907.
Tkaczuk, C., Harasimiuk, M., Król, A., and Bereś, P. K. 2015. The effect of selected pesticides on the growth of entomopathogenic fungi Hirsutella nodulosa and Beauveria bassiana. Journal of Ecological Engineering, 16(3):177-183
Wraight, S. P., Jackson, M. A., and de Kock, S. L. 2001. Production, stabilization and formulation of fungal biocontrol agents. In T. M. Butt, C. W. Jackson, and N. Magan (Eds.), Fungi as biocontrol agents: Progress, problems and potential. Wallingford, UK: CABI Publishing.