DOI: https://doi.org/10.56669/GVCB6290
ABSTRACT
Aimed to develop a new biopesticide to control the pepper root rot and root-knot nematode disease complex in Vietnam, the study focused on the identification of the new biopesticide component, the development of production technology and the evaluation of its effect on the reduction of pathogens in the pepper growing soil. The results showed, that Trichoderma, Streptomyces and Bacillus strains had the ability to inhibit pathogen fungi with a control effect of 74.3% – 100%; and that Arthrobotrys strain was able to trap nematodes with a rate of over 60% under laboratory conditions. A new biopesticide produced by mixing the biomass of selected microorganisms has a density of more than 108 CFU/g after 12 months of storage in plastic bags at room temperature. After 9 months of treatment, the new biopesticide can reduce nematode populations in pepper plantations by 81.91% at the young stage and 80.33% at the mature stage. Field demonstrations on the application of the new biopesticide showed a reduction in the population of parasitic nematodes by 75.9% - 76.8%, and the population of Phytopthora spp. by 79.7% - 80.1%. This study revealed that the new biopesticide can be used for the control of the pepper root rot and root-knot nematodes disease complex in Vietnam.
Keywords: Pepper root rot and root-knot nematodes disease complex, new biopesticide formulation, nematophagous fungi, antagonistic microorganisms.
INTRODUCTION
Pepper is one of Vietnam's key industrial crops. According to the Ministry of Agriculture and Rural Development, in 2016, the pepper production area in Vietnam was 151,900 ha with a total production of 176.6 thousand tons and an export value of US$1,422 million. Due to the low price of pepper export, unfavorable weather, climate and increasing pepper diseases, the pepper production area in Vietnam decreased to 149,800 ha in 2018 and 130,000 ha in 2021 (Plant Protection Department, 2021).
The Plant Protection Department (2010) identified the main pests of pepper root rot and root-knot nematodes disease complex including the fungi Fusarium solani, Phytophthora capsici and the nematode Meloidogyne incognita. It damaged most of the pepper growing areas in the country, especially in the Central Highlands provinces with the damaged area of nearly 13,000 ha (Plant Protection Department, 2019).
Many antagonistic microorganisms have been selected and used for the production of biopesticides to control plant pathogens fungi, including species of Trichoderma, Bacillus, Streptomyces (Ramírez-Delgado et al., 2018; Mardanova et al., 2017; Gordon, 2017; Ha Minh Thanh, 2017; Singh et al., 2014; Kestwani et al., 2013; Chung et al., 2008; Jiang et al., 2006). Using nematophagous fungi to control nematodes has already been studied. Niu and Zhang (2011), Zhang and Hyde (2014), Bakr et al. (2014), Nguyen Viet Hiep and Nguyen Thu Ha (2014) found that nematophagous fungi is a potential agent in controlling plant nematodes. The research results of Siddiqui and Akhtar (2009), Chemeltorit et al. (2016) and Gordon (2017) showed that multi-strain biopesticides are more effective than mono-strain biopesticides.
Biopesticides are one of the solutions for integrated crop pest management, which do not negatively affect the environment, human health and animals, but commercialization is very limited and in 2017, only accounted for about 5% of all pesticides applied in agriculture (Damalas and Koutroubas, 2018).
The research objective is to select and identify the microbial strains antagonistic to pathogen fungi and nematodes, to develop the biopesticide production technology and evaluate the effectiveness of the new formulation of biopesticide in controlling fungal pathogens and nematodes that caused the pepper root rot and root-knot nematodes disease complex in Vietnam.
MATERIALS AND METHODS
Nematophagous fungi Arthrobotrys oligospora NVC7.4 and antagonistic microorganisms to pathogen fungi such as Bacillus velezensis P9.1, Streptomyces enissocaesilis 2P, Trichoderma asperellum Trivass were provided by the microbial germ bank of the Soils and Fertilizers Research Institute, Plant Protection Research Institute and the Institute for Agricultural Environment in Hanoi. Pathogen microorganisms to pepper plants such as Phytophthora capsisi, Fusarium solani, Rhizoctoinia solani, Meloidogyne incognita, Meloidogyne arenaria and Rotylenchulus reniformis were provided by the Plant Protection Research Institute.
The pepper plant variety Vinhlinh was obtained from the Western Highlands Agriculture and Forestry Science Institute. A new biopesticide was produced by mixing the biomass of Trichoderma asperellum Trivass, Arthrobotrys oligospora NVC7.4, Bacillus velezensis P9.1, Streptomyces enissocaesilis 2P in w/w ration of 40% : 40% : 10% : 10%. The density of beneficial microorganisms are 108 CFU/g.
The ability of microorganisms to antagonize fungal diseases was determined by the method of diffusion of inhibitory substances in an agar medium through the size of the inhibited fungal colony (Wen – Chuan Chung et al., 2011). The activity of nematode trapping fungi was evaluated by the method of Tsay et al. (2006).
The growth conditions of selected microbial strains and their biomass production techniques to create the biopesticides were performed according to the method of Le Van Nhuong et al., (2009).
A pot experiment was carried out at the Plant Protection Research Institute and the Western Highlands Agriculture and Forestry Science Institute. The experiment includes two treatments to assess the biological efficacy of beneficial fungi against pepper plant parasitic nematodes: 1. Control infected with Meloidogyne incognita at a dose of 250 nematodes per 100 gram of soil, 2. Infected with Meloidogyne incognita and inoculated with nematophagous fungi Arthrobotrys oligospora NVC7.4 at a density of 104 CFU/g soil. Each treatment was replicated 3 times and randomized. In the pot containing 1 kilogram of methylene bromide-fumigate Rhodic Ferralsols, a pepper plant of the Vinhlinh variety was planted. The density of nematodes in the soil was monitored after 2, 4, 6 and 8 days of treatment.
The pot experiment that aims to evaluate the capability of antagonistic microorganism to control the pathogen fungi comprises of 3 treatments: 1. Control infected with the phythophthora capsisi at a density of 104 CFU/g soil , 2. Infected with the phythophthora capsisi and inoculated with Bacillus velezensis P9.1; 3. Infected with the phythophthora capsisi and inoculated with Streptomyces enissocaesilis 2P; 4. Infected with the phythophthora capsisi and inoculated with Trichoderma asperellum Trivass. The density of phythophthora capsisi used in the treatments 2, 3 and 4 are the same in terms of the control and the density of beneficial microorganisms, which are 104 CFU/g soil. The biomass of phythophthora capsisi is produced by the methods of Burgess at al. (2009) to reach the density of 106 CFU/ml and mixed with the soil in a ratio of 10 ml : 1000 g. The monitoring of the density of pathogen fungi in the soil is done after 15, 30 and 45 days of treatment.
To evaluate the effectiveness of nematophagous fungi in the control of parasitic nematode of pepper, field experiments were carried out at the young and mature stages of pepper plant. The experiment comprises of two treatments: 1. negative control through farmer practices; 2. application of the new biopesticide at a dose of 30 g/plant. The experiment was designed with 15 plants/ treatment. All experiments were laid out with 3 replications. The monitoring of the density of phythophthora pathogen fungi and nematodes in the soil is done after 1, 3, 6 and 9 months of treatment.
Field demonstrations were carried out at the 5-year old pepper plantation in Chupu - Gialai and Chukiun - Daklak. Two treatments are set up without replication, 1. Using chemical control method as part of the farmers’ practices, and 2. Application of new biopesticide with a dose of 30g/plant. Monitoring the density of pathogen fungi, nematodes in the soil is done after 0, 1 and 3 months of treatment, and calculating the control efficacy is done 3 months after treatment.
The density of nematodes in soils was determined by the method described by Ton Nguyen Tang et al., (2010). The nematodes reduction percentage and control efficacy were calculated by Henderson-Tilton’s formula (Henderson and Tilton, 1955). The density of fungal pathogens in soil was determined following Burgess et al., (2009) and the density of phythophthora spp. is evaluated by the method described by Erwin and Riberrio (1996). Study data are processed according to the IRRISTAT statistical and data processing program.
RESEARCH RESULTS
Determination of the component of a new biopesticide to control the pathogen fungi and nematode damaged pepper plants
Results of research on the determination of beneficial microorganisms in Table 1 showed, that the Trichoderma strain was highly effective in controlling Fusarium, Pythium and Rhizoctonia pathogen fungi with a control effect of 75.9% – 100%, the Streptomyces and Bacillus strains have the ability to inhibit Phytophthora pathogen fungi with the control effect of 74.3% – 75.3%.
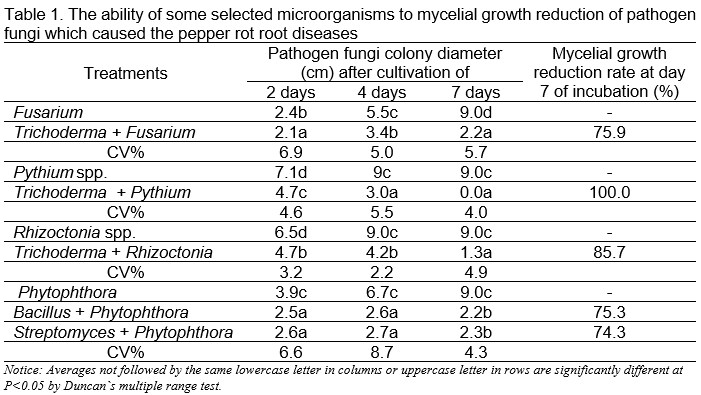
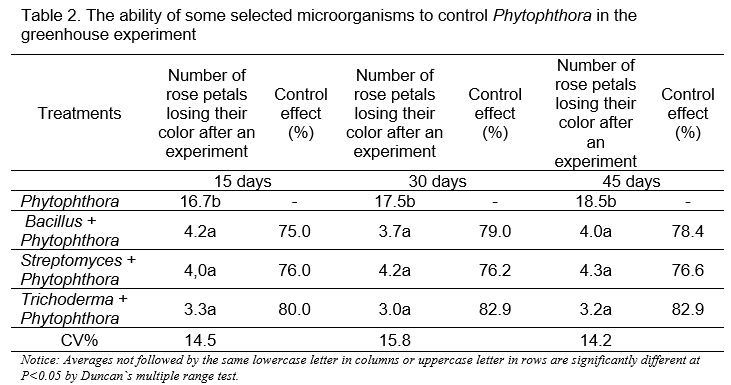
The results of the evaluation of the ability to control Phytophthora fungi in the greenhouse (Table 2) determined that the control effect of tested microorganisms to the Phytophthora population ranged from 76.6% to 82.9% after 45 days of treatments and the Trichoderma asperellum Trivass had the highest effect with the control effect of 82.9%.
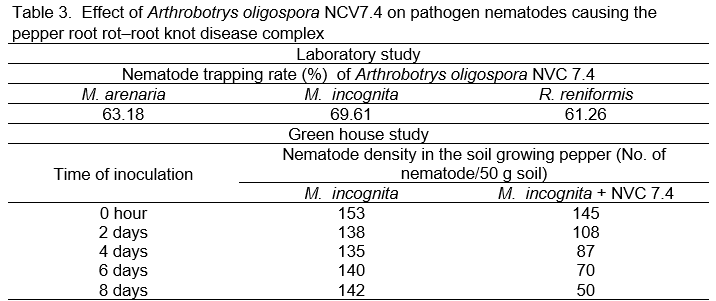
The results of evaluating the nematode trapping ability of Arthrobotrys oligospora NCV7.4 are summarized in Table 3. They showed that the used nematophagous fungi was able to trap nematodes with a rate of over 60% under laboratory conditions, and had the effect to reduce 65% of the density of nematodes in the soil in the greenhouse experiment.
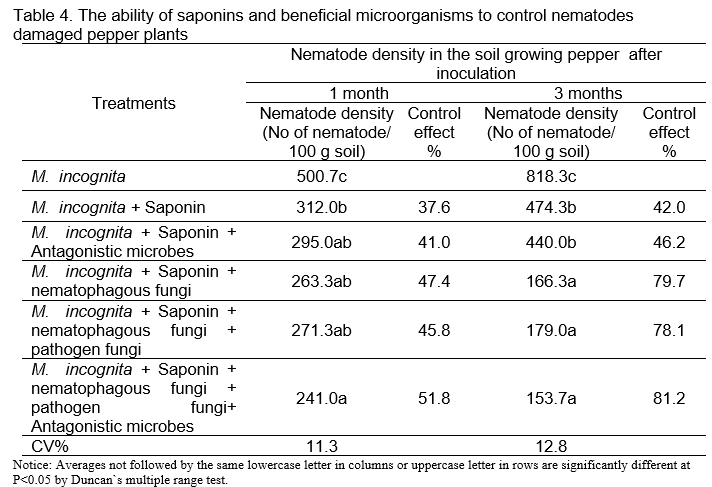
According to Ibrahim and Srour (2013), saponins are biologically active substances that have the effect of killing fungi and nematodes that damage plant roots. The results of using a mixture of antagonistic microorganisms, nematode trapping fungi and saponins in Table 4 showed that the combination of beneficial microorganisms and saponin had a higher effect in controlling pepper nematodes compared to beneficial microorganisms alone that reached 81.2% after 3 months of inoculation.
New biopesticide production
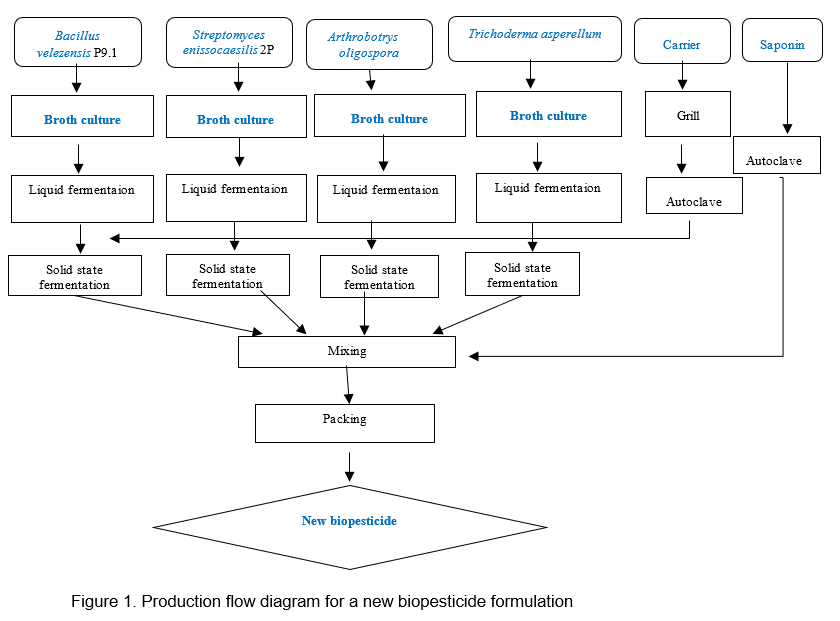
Based on previously research results on the production of biomass of selected microorganisms, a new biopesticide is produced with the density of beneficial microorganisms of 109 CFU/g after 2 steps of fermentation (liquid and solid fermentation). It takes 2 days to 7 days depending on the microorganism. The production procedure of the new biopesticide is summarized in Figure 1.
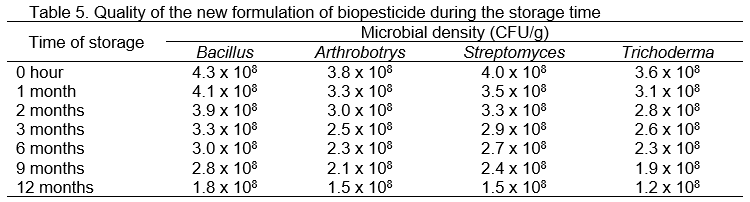
The results of quality control of the new biopesticide in Table 5, determined that after 12 months of storage in plastic bags at room temperature, the density of beneficial microorganisms was more than 108 CFU/g, in which the density of Bacillus reached 1.8 x 108 CFU/g, Arthrobotrys reached 1.5 x 108 CFU/g, Streptomyces reached 1.5 x 108 CFU/g and Trichoderma reached 1.2 x 108 CFU/g.
Field trials and demonstrations of a new biopesticide to control pathogen fungi and nematodes damaged pepper
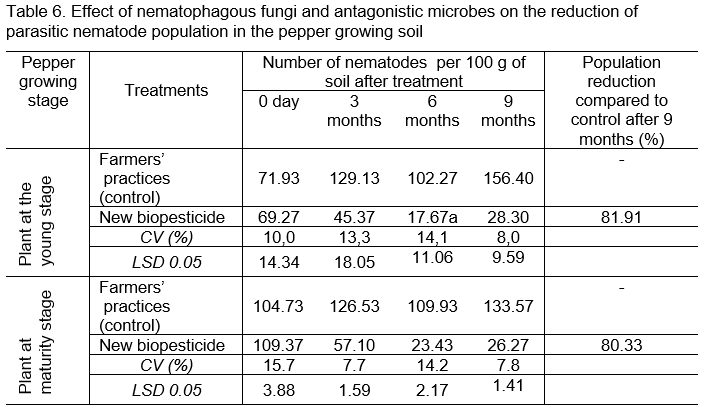
Study data on the effect of nematophagous fungi and antagonistic microorganisms on the control of parasitic nematode damaged pepper plants in Table 6 showed that nematode population were reduced by 81.91% in the pepper plantation at young stage and 80.33% at maturity stage after 9 months of treatment with the new formulation of biopesticide.
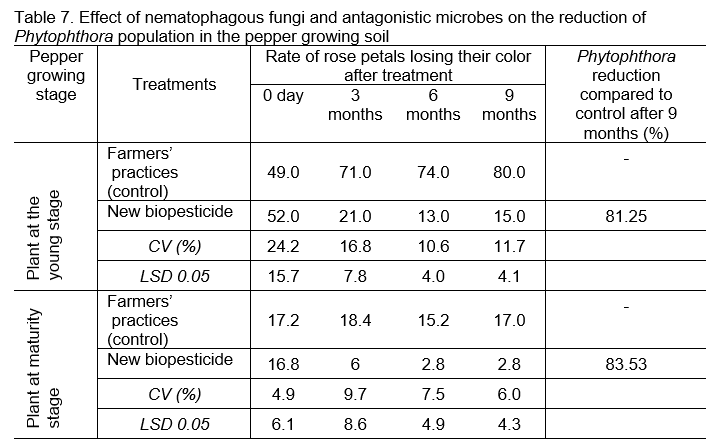
Data presented in Table 7 showed that the population of Phythophthora spp. reduced by 81.25% in the pepper plantation at young stage and 83.53% in the pepper plantation at mature stage compared to control, when the new biopesticide was applied.
The results of evaluation of the ability to control pathogen fungi and nematodes harmful to pepper in Chukiun - Daklak and Chupu - Gialai and in Figures 2 and 3 showed that after 3 months of the new biopesticide application, the control effectiveness to Phytopthora spp. is 80.1% and 79.7 %, to nematodes is 76.8% and 75.9%, respectively.
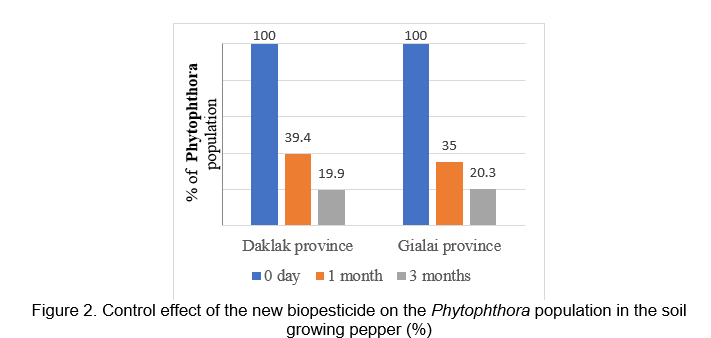
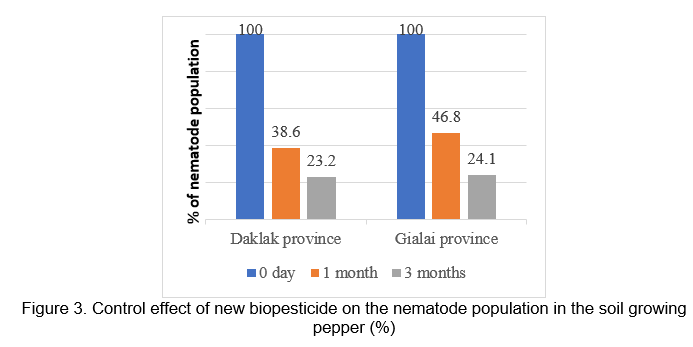
DISCUSSION AND CONCLUSION
Streptomycetes are endogenous microorganisms that have received much research attention recently for the purpose of biological control of plant diseases (Bubici, 2018). Many strains of Streptomyces have been studied to evaluate their ability to control Phytophthora capsici, which caused disease in peppers, and tomatoes with control efficiency reached over 70% in greenhouse conditions (Ezziyyani et al., 2007). Several biological products derived from Streptomyces spp. have been commercialized such as Mycostop produced from S. griseoviridis K61 in Finland, and Germany; Actinovate®, Micro108®, Action Iron® produced from S. Lydicus in the United States of America; or YAN TEN manufactured from S. saraceticus KH400 in Taiwan. In Vietnam, there has been a lot of research on the application of actinomycetes to control plant diseases and initially isolated and selected some Streptomycetes capable of antagonizing the Phytophthora spp. causing lotus stem rot (Dinh Hong Thai and Le Minh Tuong, 2016), F. oxysporum harmful to tomato (Nguyen Thi Kim Cuc et al. 2014)... Truong Thanh Thao et al. (2019) isolated and selected 6 Streptomycetes strains that biosynthesize chitinase and protease enzymes capable of killing the nematode Pratylenchus spp.
Bacillus exists naturally in soil, water, and air, and they are capable of spore forming and surviving in difficult conditions. Many Bacillus species have the ability to biosynthesize extracellular enzymes such as amylase, pullulanase, β-glucanase, β-galactosidase, cellulase, xylanase, chitinase, esterase, lipase and bacteriocins that inhibit the growth of pathogenic microorganisms (Siahmoshteh et al., 2019; Cheng et al., 2019; Keswani et al., 2019). According to Jang et al., (2006); Chung et al., (2008); Mardanova et al., (2017) many Bacillus strains have the ability to antagonize and eliminate the fungal diseases of pepper and vegetables. In Vietnam, research on using Bacillus as a biological agent to control plant pathogens and nematodes has been published by several authors (Pham Viet Cuong et al., 2003; Vu Thuy Nga, 2008; Tran Thi Phuong Hanh et al., 2019; Trinh Thi Huyen Trang et al., 2019).
Trichoderma has the ability to antagonize many fungal species and plant nematodes through parasitic mechanisms, synthesizing enzymes (cellulase, glucanase, chitinase. v.v.) or synthesizing antibiotics, and nutritional competition (Harman, 2006; Keswani et al., 2013, 2014; Singh et al., 2017). Research by Ezziyyanil et al. (2007) determined that the mixture of Trichoderma harzianum and Streptomyces rochei could reduce up to 75% the harmfulness of P. capcisi. Many studies confirm that Trichoderma has the ability to enhance plant growth and development through the effect of stimulating the formation and stronger development of plant roots. Trichoderma through extracellular enzymes, has the ability to degrade organic compounds in the soil, contributing to the increase of soil fertility. In Vietnam, a number of published studies have shown that Trichoderma is a potential biological agent in controlling fungal diseases and nematodes (Dang Thuy Linh and Nguyen Van Hoa, 2004; Tran Kim Loang, 2007; Nguyen Tang Ton et al. 2010; Le Van Trinh 2010; Le Duc Khanh, 2015; Ha Minh Thanh, 2017).
Nematophagous fungi played an important role in the rhizosphere of agricultural plants (Usman and Siddiqui 2012; Singh et al., 2013). Arthrobotrys oligospora is well studied as a biocontrol agent due to the presence of variety of trapping structures with functional nematode‐capturing devices (Khan et al., 2011; Simon and Anamika, 2011; Bakr et al., 2014). Singh et al. (2012) reported that Arthrobotrys oligospora has the ability to trap and kill the nematode Meloidogyne graminicola and Rhizoctonia solani and has the potential to reduce the number of knot 57.6 - 62.0%, reduced disease 55.7 - 59.3%. In Vietnam, Nguyen Viet Hiep and Nguyen Thu Ha (2014); Nguyen Viet Hiep et al. (2019) announced that nematophagous fungi from coffee and pepper growing soils as having the trapping activity against a wide range of nematodes, especially the black pepper nematodes as Meloidogyne arenaria, Meloidogyne incognita, and the coffee nematodes as Pratylenchus coffea. The nematode trapping efficiency reached in laboratory conditions 51.36% - 72.37 %, in green houses 46.46% - 74.29% and in the field 49.45% - 67.31 %.
Biopesticides are produced from biological control agents, which may include live microorganisms (bacteria, fungi, actinomycetes, viruses, and protozoa), biologically active compounds (spinosads) and avermectins), or naturally derived materials such as plant extracts (Kiewnick, 2007). After a long time of development, biological pesticides tend to shift from using one biological agent to a combination of many different biological agents based on the studies of interactions between plants, pathogens, biological control agents, plant rhizosphere microbes, and the environment (Grosch et al., 2012). Singh et al. (2012) proposed that biopesticide components should be based on the synergistic action of biological control agents.
Balog et al. (2017) reported that the global value of the biopesticide market in 2017 increased by about 16% and reached a value of about US$10 billion worldwide. Kumar and Singh (2015) reported that globally, there are about 300 commercialized biopesticide products, and the trend is increasing by about 10% per year. Olson (2015) predicts that by the late 2040s and early 2050s, biopesticides will be at par with chemical pesticides in terms of market size. Biopesticides have the advantage of being less detrimental to the environment, providing more specific pest control, and being effective in small amounts, leaving no problematic residues. Biopesticides are one of the important components of integrated pest management programs - IPM (Damalas and Koutroubas, 2018).
New biopesticide formulation is produced by mixing the biomass of Trichoderma asperellum Trivass, Arthrobotrys oligospora NVC7.4, Bacillus velezensis P9.1 and Streptomyces enissocaesilis 2P had a density of beneficial microorganisms of more than 108 CFU/g after 12 months of storage at room temperature and had a positive effect on reducing the population of pathogenic fungi and nematodes in the pepper growing soil in Vietnam. The field trial with the new biopesticide formulation showed that the nematode population was reduced by 81.91 % in the pepper plantation at young stage and 80.33% at mature stage after 9 months of treatment. The same trend can be seen in the field demonstration, where the control effectiveness of Phytopthora spp., and nematodes is 79.7%-80.1% and 75.9%-76.8% respectively, after 3 months of inoculation. This study revealed that a new formulation can be used as biocontrol for pepper root rot and root-knot nematode disease complex in Vietnam.
REFERENCES
Bakr, R.A., Mahdy, M. E. and Mousa, E.M. 2014. Biological control of root knot nematode meloidogyne incognita by Arthrobotrys oligospora. Egyptian Journal of Crop Protection, 9(1), 1-11.
Balog A., Hartel T., Loxdale HD., Wilson K. 2017. Differences in the progress of the biopesticide revolution between the EU and other major crop-growing regions. Pest Manag. Sci. 2017, 73, 2203–2208.
Bubici, G. 2018. Streptomyces spp. as biocontrol agents against Fusarium species. CAB Reviews 13, No. 050.
Burgess, LW., Knight, TE., Tesoriero, L. and Phan Thuy Hien. 2009. Diagnostic manual for plant diseases in Vietnam. Australian Center for International Agricultural Research – ACIAR (In Vietnamese)
Chemeltorit PP, Mutaqin KH, Widodo W. 2017. Combining Trichoderma hamatum THSW13 and Pseudomonas aeruginosa BJ10–86: a synergistic chili pepper seed treatment for Phytophthora capsici infested soil. Eur J Plant Pathol.147(1):157–166.
Cheng, X., X. Ji, Y. Ge, J. Li, W. Qi and K. Qiao. 2019. Characterization of Antagonistic Bacillusmethylotrophicus isolated from rhizosphere and its biocontrol effects on maize stalk rot. Phytopathology,109 (4): 571-581.
Chung S., Kong H., Buyer JS., Lakshman DK., Lydon J., Kim SD. 2008. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl Microbiol Biotechnol, 80 (1), 115-123
Nguyen Thi Kim Cuc, Tran thi Hong, Pham Thi Thuy Hoai, Pham Viet Cuong. 2014. Isolation of microorganisms antagonistic to plant pathogen fungi and evaluation the biological activity invitro. Science and technology journal 52 (4), 419-430 (In Vietnamese with English abstract)
Pham Viet Cuong, Pham van Toan, Nguyen thi Tuyet Mai, Nguyen Thi Kim Cuc. 2002. Indol-acetic-acid production of Bacillus isolation from soil in Vietnam. Proceeding of national conference on the biotechnology, Hanoi 12/2003, 213-217 (In Vietnamese with English abstract)
Damalas and Koutroubas, SD. 2018. Current Status and Recent Developments in Biopesticide Use. Agriculture 2018, 8, 13; doi:10.3390/agriculture8010013
Department of Crop Production. 2021. Summary report of 2021 and implementation of the 2022 plan (In Vietnamese).
Department of Plant Protection. 2010. List of harmful organisms on some crops and post-harvest plant products in Vietnam. Agricultural publishing house 2010 (In Vietnamese)
Department of Plant Protection. 2019. Report on the current production and proposal for sustainable pepper production in Vietnam (In Vietnamese)
Erwin, D. C., and Ribeiro, O. K. 1996. Phytophthora Diseases Worldwide. American Phytopathological Society Press, St. Paul, MN.
Ezziyyani, M., Requena, ME., Egea-Gilabert, C., Candela, ME. 2007. Biological Control of Phytophthora Root Rot of Pepper Using Trichoderma harzianum and Streptomyces rochei in Combination. J Phytopathol. junho de 155(6):342–349.
Gordon T.R. 2017. Fusarium oxysporum and the Fusarium wilt syndrome. Annual Review of Phytopathology 55(1):23–39.
Grosch R., Dealtry S., Schreiter S., Berg G., Mendonça-Hagler L., Smalla K. 2012. Biocontrol of Rhizoctonia solani: complex interaction of biocontrol strains, pathogen and indigenous microbial community in the rhizosphere of lettuce shown by molecular methods. Plant Soil 361 343–357. 10.1007/s11104-012-1239
Hanh Tran Thi Phuong, San-Lang Wang, Van Bon Nguyen, Dinh Minh Tran, Dinh Sy Nguyen and Anh Dzung Nguyen. 2019. Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam. Agronomy 9, 714; doi:10.3390/agronomy9110714
Harman GE. 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96:190-194.
Henderson, CF. and Tilton, EW. 1955. Test with acaricides against the brow wheat mite. J. Econ.Entomol.48: 157-161
Nguyen Viet Hiep, Nguyen Thu Ha. 2014. Study on the nematode trapping ability of some ring fungus strains isolated from coffee and pepper growing soils in Vietnam. Vietnam Journal of Agricultural Science and Technology, No. 4(50), p. 90 – 96 (In Vietnamese with English abstract)
Nguyen Viet Hiep, Nguyen Thu Ha, Tran Thi Thanh Thuy and Pham Van Toan. 2019. Isolation and selection of Arthrobotrys nematophagous fungi to control the nematodes on coffee and black pepper plants in Vietnam. Archives of Phytopathology and Plant Protection Volume 52 Numbers 7–8: 825-843
Ibrahim MAR, Srour HAM (2013). Saponins Suppress Nematode Cholesterol Biosynthesis and Inhibit Root Knot Nematode Development in Tomato Seedlings. Nat Prod Chem Res 2: 123 doi:10.4172/ 2329-6836.1000123.
Jiang Z. Q., Guo Y. H., Li S. M., Qi H. Y., Guo J. H. 2006. Evaluation of biocontrol effciency of Bacillus preparations and application methods against Phytophthora blight of bell pepper. Biological Control 36. 216–223.
Khan, T., Shadab, S., Afroz, R., Aziz, M.A. and Farooqui, M. 2011. Study of suppressive effects of biological agent fungus, natural organic compounds and carbofuron on root knot nematodes of tomato (Lycopersicon esculentum). J Microbiol Biotechnol Rev 1, 7– 11.
Le Duc Khanh. 2015. Report on scientific and technological results of the project “Research on nematode damaged pepper, coffee plant and technology for the effective control in the main cultivation area“ (In Vietnamese).
Keswani, C., Singh, S.P. and Singh, H.B. 2013. A superstar in biocontrol enterprise: Trichoderma spp. Biotech Today 3, 27–30.
Keswani C., Mishra S., Sarma B.K., Singh S.P. and Singh HB. 2014. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Applied Microbiology and Biotechnology 98, 533–544.
Keswani C., Singh HB., García-Estrada C., Caradus J., He YW., Aichour SM., Glare TR., Borriss R., Sansinenea E. 2019. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Applied Microbiology and Biotechnology.
Kiewnick S. 2007. Practicalities of developing and registering microbial biological control agents. CAB Rev. 2 1–11. 10.1079/PAVSNNR20072013
Kumar S. and Singh A. 2015. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 2015, 6, e129
Dang Thuy Linh and Nguyen Van Hoa. 2004. Antagonistic activity and growth parameters of 3 trichoderma strains and 3 Phytophthora strains isolated from durian, longan trees. Scientific report of southern horticultural research institute (In Vietnamese with English abstract)
Tran Kim Loang. 2007. Study on production of Trichoderma preparation to controlling some pathogen fungi damaged Cacao, durian and pepper in highland Taynguyen. Scientific report of Western Highlands Agriculture and Forestry Science Institute (In Vietnamese with English abstract) .
Mardanova, A.M., Hadieva, G.F., Lutfullin, M.T., Khilyas, I.V., Minnullina, L.F., Gilyazeva, A.G., Bogomolnaya, L.M. and Sharipova, M.R. 2017. Bacillus subtilis strains with antifungal activity against the phytopathogenic Fungi. Agricultural Sciences, 8, 1-20.
Vu Thuy Nga. 2008. Isolation and selection of Bacillus antagonistic to Fusarium oxysporum damaged vegetable. Agricultural science and technology journal (In Vietnamese with English abstract)
Le Van Nhuong, Nguyen Van Cach, Quan Le Ha, Tran Lien Ha, Nguyen Thanh Hang, Hoang Dinh Hoa, Nguyen Lan Huong, Ngo Thi Mai, Dinh Kim Nhung, Khuat Huu Thanh, Nguyen Quang Thao, Pham Thi Thuy, Pham van Toan. 2009. Basic of biotechnology. Vol 4. Microbial technology. Education publishing house (In Vietnamese)
Niu,.XM. and Ke-Qin Zhang, KQ. 2011. Arthrobotrys oligospora: a model organism for understanding the interaction between fungi and nematodes, Mycology, 2:2, 59-78, DOI: 10.1080/21501203.2011.562559
Olson S. 2015. An analysis of the biopesticide market now and where is going. Outlooks Pest Manag. 26, 203–206.
Ramírez-Delgado, E., Luna-Ruíz1, J. J., Moreno-Rico, O., Quiroz-Velásquesz, J. D. C. and Hernández-Mendoza, J. L. 2018. Effect of Trichoderma on Growth and Sporangia Production of Phytophthora capsici. Journal of Agricultural Science, Vol. 10, No. 6, 8-15
Siahmoshteh, F., Z. Hamidi-Esfahani, D. Spadaro, M. Shams-Ghahfarokhi and M. Razzaghi-Abyaneh. 2019. Unraveling the mechanism of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens against aflatoxigenic Aspergillus parasiticus. 89: 300-307.
Siddiqui, Z. A. and Akhtar, M. S. 2009. Effects of antagonistic fungi and plant growth-promoting rhizobacteria on growth of tomato and reproduction of the root-knot nematode, Meloidogyne incognita. Australasian Plant Pathology, 2009, 38, 22–28.
Simon, S. and Anamika. 2011. Management of root knot diseases in rice caused by Meloidogyne graminicola through nematophagous fungi. Indian J Agric Sci 3, 122– 127.
Singh, U.B., Sahu, A.,Sahu,N., Singh, R.K. Renu, S., Singh, D.P., Manna, M.C., Sarma, B.K., Singh, H.B.,Singh, K.P. 2012. Arthrobotrys oligospora mediated biological control of diseases of tomato (Lycopersicon esculentum Mill.) caused by Meloidogyne incognita and Rhizoctonia solani. https://doi.org/10.1111/jam.12009.
Singh, U.B., Sahu, A., Sahu, N. 2013. Nematophagous fungi: Catenaria anguillulae and Dactylaria brochopaga from seed galls as potential biocontrol agents of Anguina tritici and Meloidogyne graminicola in wheat (Triticum aestivum L.). Biological Control, 67: 475-482.
Singh, H.B., Singh, A. and Sarma, B.K. 2014. Trichoderma viride 2 % WP (Strain No. BHU-2953) formulation suppresses tomato wilt caused by Fusarium oxysporum f. sp. lycopersici and chilli damping-off caused by Pythium aphanidermatum effectively under different agroclimatic conditions. International Journal of Agriculture, Environment and Biotechnology 7, 313–320.
Singh V., Ray S., Bisen K., Keswani C., Upadhyay RS., Sarma BK., Singh HB. 2017. Unravelling the Dual Applications of Trichoderma spp. as Biopesticide and Biofertilizer. In Advances in PGPR Research eds HB. Singh, BK. Sarma and C. Keswani. CAB International 2017, 264-274
Dinh Hong Thai and Le Minh Tuong. 2016. Survey the capability of streptomyces antagonism to Phytophthora sp. Damaged lottus plant. Scientific journal of Cantho university, 20-27 (In Vietnamese with English abstract)
Ha Minh Thanh. 2017. Report on scientific and technological results of the project “Improving production technology and application of probiotics to prevent fast and slow death on pepper plants (In Vietnamese)
Truong Thanh Thao, Vo Quoc Canh, Nguyen Thi Thu Nga. 2019. Isolation and selection of potential streptomyces strains antagonistic to Pratylenchus sp. In the laboratory. Scientific journal of Cantho university 55(2B), 19-27 (In Vietnamese with English abstract)
Nguyen Tang Ton, Nguyen Thi Huong, Le Quang Nhut, Nguyen Mong, Do Trung Binh, Nguyen Luong Thien, Do Dinh Dan, La Pham Lan, Doan Van Trung, Tran Kim Loang, Tran thi Thu Ha. 2010. Report on scientific and technological results of the project “Research on the intergrated management of soilborn diseases on the pepper” (In Vietnamese)
Trinh Thi Huyen Trang, San‑Lang Wang, Van Bon Nguyen, Minh Dinh Tran, Chien Thang Doan, Thi Phuong Khanh Vo, Que V. Huynh, Anh Dzung Nguyen. 2019. A potent antifungal rhizobacteria Bacillus velezensis RB.DS29 isolated from black pepper (Piper nigrumL.). Research on Chemical Intermediates 45:5309–5323, https://doi.org/ 10.1007/s11164-019-03971-5
Le Van Trinh. 2010. Report on scientific and technological results of the project “Research on the development of biopreparation to control pathogen fungi and nematodes damaged pepper” (In Vietnamese)
Tsay T. T., Chen P. C., Wu, W. S. 2006. A New Method for Isolating and Selecting Agents with High Antagonistic Ability Against Plant Parasitic Nematodes. Plant Pathology Bulletin 15: 9-16.
Usman, A. and Siddiqui, M.A. 2012. Effect of some fungal strains for the management of root-knot nematode (Meloidogyne in-cognita) on eggplant (Solanum melongena). Journal of Agriculture Tech-nology, 8(1): 213-218.
Wen-Chuan Chung, Rey-Shung Wu, Chia-Ping Hsu, Hung-Chang Huang, Jenn-Wen Huang. 2011. Application of antagonistic rhizobacteria for control of Fusarium seedling blight and basal rot of lily. Australasian Plant Pathol. (2011) 40:269–276
Zhang, K.Q., Hyde. K.D. 2014. Nematode-Trapping Fungi. Fungal Diversity Research Series. Volume 23. Springer Dordrecht Heidelberg New York London.
Study on the Development of New Biopesticide to Control Pathogen Fungi and Nematode Damaged Black Pepper in Vietnam
DOI: https://doi.org/10.56669/GVCB6290
ABSTRACT
Aimed to develop a new biopesticide to control the pepper root rot and root-knot nematode disease complex in Vietnam, the study focused on the identification of the new biopesticide component, the development of production technology and the evaluation of its effect on the reduction of pathogens in the pepper growing soil. The results showed, that Trichoderma, Streptomyces and Bacillus strains had the ability to inhibit pathogen fungi with a control effect of 74.3% – 100%; and that Arthrobotrys strain was able to trap nematodes with a rate of over 60% under laboratory conditions. A new biopesticide produced by mixing the biomass of selected microorganisms has a density of more than 108 CFU/g after 12 months of storage in plastic bags at room temperature. After 9 months of treatment, the new biopesticide can reduce nematode populations in pepper plantations by 81.91% at the young stage and 80.33% at the mature stage. Field demonstrations on the application of the new biopesticide showed a reduction in the population of parasitic nematodes by 75.9% - 76.8%, and the population of Phytopthora spp. by 79.7% - 80.1%. This study revealed that the new biopesticide can be used for the control of the pepper root rot and root-knot nematodes disease complex in Vietnam.Keywords: Pepper root rot and root-knot nematodes disease complex, new biopesticide formulation, nematophagous fungi, antagonistic microorganisms.INTRODUCTION
Pepper is one of Vietnam's key industrial crops. According to the Ministry of Agriculture and Rural Development, in 2016, the pepper production area in Vietnam was 151,900 ha with a total production of 176.6 thousand tons and an export value of US$1,422 million. Due to the low price of pepper export, unfavorable weather, climate and increasing pepper diseases, the pepper production area in Vietnam decreased to 149,800 ha in 2018 and 130,000 ha in 2021 (Plant Protection Department, 2021).The Plant Protection Department (2010) identified the main pests of pepper root rot and root-knot nematodes disease complex including the fungi Fusarium solani, Phytophthora capsici and the nematode Meloidogyne incognita. It damaged most of the pepper growing areas in the country, especially in the Central Highlands provinces with the damaged area of nearly 13,000 ha (Plant Protection Department, 2019).
Many antagonistic microorganisms have been selected and used for the production of biopesticides to control plant pathogens fungi, including species of Trichoderma, Bacillus, Streptomyces (Ramírez-Delgado et al., 2018; Mardanova et al., 2017; Gordon, 2017; Ha Minh Thanh, 2017; Singh et al., 2014; Kestwani et al., 2013; Chung et al., 2008; Jiang et al., 2006). Using nematophagous fungi to control nematodes has already been studied. Niu and Zhang (2011), Zhang and Hyde (2014), Bakr et al. (2014), Nguyen Viet Hiep and Nguyen Thu Ha (2014) found that nematophagous fungi is a potential agent in controlling plant nematodes. The research results of Siddiqui and Akhtar (2009), Chemeltorit et al. (2016) and Gordon (2017) showed that multi-strain biopesticides are more effective than mono-strain biopesticides.Biopesticides are one of the solutions for integrated crop pest management, which do not negatively affect the environment, human health and animals, but commercialization is very limited and in 2017, only accounted for about 5% of all pesticides applied in agriculture (Damalas and Koutroubas, 2018).The research objective is to select and identify the microbial strains antagonistic to pathogen fungi and nematodes, to develop the biopesticide production technology and evaluate the effectiveness of the new formulation of biopesticide in controlling fungal pathogens and nematodes that caused the pepper root rot and root-knot nematodes disease complex in Vietnam.MATERIALS AND METHODS
The pepper plant variety Vinhlinh was obtained from the Western Highlands Agriculture and Forestry Science Institute. A new biopesticide was produced by mixing the biomass of Trichoderma asperellum Trivass, Arthrobotrys oligospora NVC7.4, Bacillus velezensis P9.1, Streptomyces enissocaesilis 2P in w/w ration of 40% : 40% : 10% : 10%. The density of beneficial microorganisms are 108 CFU/g.The ability of microorganisms to antagonize fungal diseases was determined by the method of diffusion of inhibitory substances in an agar medium through the size of the inhibited fungal colony (Wen – Chuan Chung et al., 2011). The activity of nematode trapping fungi was evaluated by the method of Tsay et al. (2006).The growth conditions of selected microbial strains and their biomass production techniques to create the biopesticides were performed according to the method of Le Van Nhuong et al., (2009).
A pot experiment was carried out at the Plant Protection Research Institute and the Western Highlands Agriculture and Forestry Science Institute. The experiment includes two treatments to assess the biological efficacy of beneficial fungi against pepper plant parasitic nematodes: 1. Control infected with Meloidogyne incognita at a dose of 250 nematodes per 100 gram of soil, 2. Infected with Meloidogyne incognita and inoculated with nematophagous fungi Arthrobotrys oligospora NVC7.4 at a density of 104 CFU/g soil. Each treatment was replicated 3 times and randomized. In the pot containing 1 kilogram of methylene bromide-fumigate Rhodic Ferralsols, a pepper plant of the Vinhlinh variety was planted. The density of nematodes in the soil was monitored after 2, 4, 6 and 8 days of treatment.
The pot experiment that aims to evaluate the capability of antagonistic microorganism to control the pathogen fungi comprises of 3 treatments: 1. Control infected with the phythophthora capsisi at a density of 104 CFU/g soil , 2. Infected with the phythophthora capsisi and inoculated with Bacillus velezensis P9.1; 3. Infected with the phythophthora capsisi and inoculated with Streptomyces enissocaesilis 2P; 4. Infected with the phythophthora capsisi and inoculated with Trichoderma asperellum Trivass. The density of phythophthora capsisi used in the treatments 2, 3 and 4 are the same in terms of the control and the density of beneficial microorganisms, which are 104 CFU/g soil. The biomass of phythophthora capsisi is produced by the methods of Burgess at al. (2009) to reach the density of 106 CFU/ml and mixed with the soil in a ratio of 10 ml : 1000 g. The monitoring of the density of pathogen fungi in the soil is done after 15, 30 and 45 days of treatment.
To evaluate the effectiveness of nematophagous fungi in the control of parasitic nematode of pepper, field experiments were carried out at the young and mature stages of pepper plant. The experiment comprises of two treatments: 1. negative control through farmer practices; 2. application of the new biopesticide at a dose of 30 g/plant. The experiment was designed with 15 plants/ treatment. All experiments were laid out with 3 replications. The monitoring of the density of phythophthora pathogen fungi and nematodes in the soil is done after 1, 3, 6 and 9 months of treatment.
Field demonstrations were carried out at the 5-year old pepper plantation in Chupu - Gialai and Chukiun - Daklak. Two treatments are set up without replication, 1. Using chemical control method as part of the farmers’ practices, and 2. Application of new biopesticide with a dose of 30g/plant. Monitoring the density of pathogen fungi, nematodes in the soil is done after 0, 1 and 3 months of treatment, and calculating the control efficacy is done 3 months after treatment.
The density of nematodes in soils was determined by the method described by Ton Nguyen Tang et al., (2010). The nematodes reduction percentage and control efficacy were calculated by Henderson-Tilton’s formula (Henderson and Tilton, 1955). The density of fungal pathogens in soil was determined following Burgess et al., (2009) and the density of phythophthora spp. is evaluated by the method described by Erwin and Riberrio (1996). Study data are processed according to the IRRISTAT statistical and data processing program.
RESEARCH RESULTS
The results of the evaluation of the ability to control Phytophthora fungi in the greenhouse (Table 2) determined that the control effect of tested microorganisms to the Phytophthora population ranged from 76.6% to 82.9% after 45 days of treatments and the Trichoderma asperellum Trivass had the highest effect with the control effect of 82.9%.According to Ibrahim and Srour (2013), saponins are biologically active substances that have the effect of killing fungi and nematodes that damage plant roots. The results of using a mixture of antagonistic microorganisms, nematode trapping fungi and saponins in Table 4 showed that the combination of beneficial microorganisms and saponin had a higher effect in controlling pepper nematodes compared to beneficial microorganisms alone that reached 81.2% after 3 months of inoculation.New biopesticide productionField trials and demonstrations of a new biopesticide to control pathogen fungi and nematodes damaged pepperStudy data on the effect of nematophagous fungi and antagonistic microorganisms on the control of parasitic nematode damaged pepper plants in Table 6 showed that nematode population were reduced by 81.91% in the pepper plantation at young stage and 80.33% at maturity stage after 9 months of treatment with the new formulation of biopesticide.Data presented in Table 7 showed that the population of Phythophthora spp. reduced by 81.25% in the pepper plantation at young stage and 83.53% in the pepper plantation at mature stage compared to control, when the new biopesticide was applied.
DISCUSSION AND CONCLUSION
Streptomycetes are endogenous microorganisms that have received much research attention recently for the purpose of biological control of plant diseases (Bubici, 2018). Many strains of Streptomyces have been studied to evaluate their ability to control Phytophthora capsici, which caused disease in peppers, and tomatoes with control efficiency reached over 70% in greenhouse conditions (Ezziyyani et al., 2007). Several biological products derived from Streptomyces spp. have been commercialized such as Mycostop produced from S. griseoviridis K61 in Finland, and Germany; Actinovate®, Micro108®, Action Iron® produced from S. Lydicus in the United States of America; or YAN TEN manufactured from S. saraceticus KH400 in Taiwan. In Vietnam, there has been a lot of research on the application of actinomycetes to control plant diseases and initially isolated and selected some Streptomycetes capable of antagonizing the Phytophthora spp. causing lotus stem rot (Dinh Hong Thai and Le Minh Tuong, 2016), F. oxysporum harmful to tomato (Nguyen Thi Kim Cuc et al. 2014)... Truong Thanh Thao et al. (2019) isolated and selected 6 Streptomycetes strains that biosynthesize chitinase and protease enzymes capable of killing the nematode Pratylenchus spp.Bacillus exists naturally in soil, water, and air, and they are capable of spore forming and surviving in difficult conditions. Many Bacillus species have the ability to biosynthesize extracellular enzymes such as amylase, pullulanase, β-glucanase, β-galactosidase, cellulase, xylanase, chitinase, esterase, lipase and bacteriocins that inhibit the growth of pathogenic microorganisms (Siahmoshteh et al., 2019; Cheng et al., 2019; Keswani et al., 2019). According to Jang et al., (2006); Chung et al., (2008); Mardanova et al., (2017) many Bacillus strains have the ability to antagonize and eliminate the fungal diseases of pepper and vegetables. In Vietnam, research on using Bacillus as a biological agent to control plant pathogens and nematodes has been published by several authors (Pham Viet Cuong et al., 2003; Vu Thuy Nga, 2008; Tran Thi Phuong Hanh et al., 2019; Trinh Thi Huyen Trang et al., 2019).Trichoderma has the ability to antagonize many fungal species and plant nematodes through parasitic mechanisms, synthesizing enzymes (cellulase, glucanase, chitinase. v.v.) or synthesizing antibiotics, and nutritional competition (Harman, 2006; Keswani et al., 2013, 2014; Singh et al., 2017). Research by Ezziyyanil et al. (2007) determined that the mixture of Trichoderma harzianum and Streptomyces rochei could reduce up to 75% the harmfulness of P. capcisi. Many studies confirm that Trichoderma has the ability to enhance plant growth and development through the effect of stimulating the formation and stronger development of plant roots. Trichoderma through extracellular enzymes, has the ability to degrade organic compounds in the soil, contributing to the increase of soil fertility. In Vietnam, a number of published studies have shown that Trichoderma is a potential biological agent in controlling fungal diseases and nematodes (Dang Thuy Linh and Nguyen Van Hoa, 2004; Tran Kim Loang, 2007; Nguyen Tang Ton et al. 2010; Le Van Trinh 2010; Le Duc Khanh, 2015; Ha Minh Thanh, 2017).
Nematophagous fungi played an important role in the rhizosphere of agricultural plants (Usman and Siddiqui 2012; Singh et al., 2013). Arthrobotrys oligospora is well studied as a biocontrol agent due to the presence of variety of trapping structures with functional nematode‐capturing devices (Khan et al., 2011; Simon and Anamika, 2011; Bakr et al., 2014). Singh et al. (2012) reported that Arthrobotrys oligospora has the ability to trap and kill the nematode Meloidogyne graminicola and Rhizoctonia solani and has the potential to reduce the number of knot 57.6 - 62.0%, reduced disease 55.7 - 59.3%. In Vietnam, Nguyen Viet Hiep and Nguyen Thu Ha (2014); Nguyen Viet Hiep et al. (2019) announced that nematophagous fungi from coffee and pepper growing soils as having the trapping activity against a wide range of nematodes, especially the black pepper nematodes as Meloidogyne arenaria, Meloidogyne incognita, and the coffee nematodes as Pratylenchus coffea. The nematode trapping efficiency reached in laboratory conditions 51.36% - 72.37 %, in green houses 46.46% - 74.29% and in the field 49.45% - 67.31 %.Biopesticides are produced from biological control agents, which may include live microorganisms (bacteria, fungi, actinomycetes, viruses, and protozoa), biologically active compounds (spinosads) and avermectins), or naturally derived materials such as plant extracts (Kiewnick, 2007). After a long time of development, biological pesticides tend to shift from using one biological agent to a combination of many different biological agents based on the studies of interactions between plants, pathogens, biological control agents, plant rhizosphere microbes, and the environment (Grosch et al., 2012). Singh et al. (2012) proposed that biopesticide components should be based on the synergistic action of biological control agents.Balog et al. (2017) reported that the global value of the biopesticide market in 2017 increased by about 16% and reached a value of about US$10 billion worldwide. Kumar and Singh (2015) reported that globally, there are about 300 commercialized biopesticide products, and the trend is increasing by about 10% per year. Olson (2015) predicts that by the late 2040s and early 2050s, biopesticides will be at par with chemical pesticides in terms of market size. Biopesticides have the advantage of being less detrimental to the environment, providing more specific pest control, and being effective in small amounts, leaving no problematic residues. Biopesticides are one of the important components of integrated pest management programs - IPM (Damalas and Koutroubas, 2018).New biopesticide formulation is produced by mixing the biomass of Trichoderma asperellum Trivass, Arthrobotrys oligospora NVC7.4, Bacillus velezensis P9.1 and Streptomyces enissocaesilis 2P had a density of beneficial microorganisms of more than 108 CFU/g after 12 months of storage at room temperature and had a positive effect on reducing the population of pathogenic fungi and nematodes in the pepper growing soil in Vietnam. The field trial with the new biopesticide formulation showed that the nematode population was reduced by 81.91 % in the pepper plantation at young stage and 80.33% at mature stage after 9 months of treatment. The same trend can be seen in the field demonstration, where the control effectiveness of Phytopthora spp., and nematodes is 79.7%-80.1% and 75.9%-76.8% respectively, after 3 months of inoculation. This study revealed that a new formulation can be used as biocontrol for pepper root rot and root-knot nematode disease complex in Vietnam.REFERENCES
Bakr, R.A., Mahdy, M. E. and Mousa, E.M. 2014. Biological control of root knot nematode meloidogyne incognita by Arthrobotrys oligospora. Egyptian Journal of Crop Protection, 9(1), 1-11.
Balog A., Hartel T., Loxdale HD., Wilson K. 2017. Differences in the progress of the biopesticide revolution between the EU and other major crop-growing regions. Pest Manag. Sci. 2017, 73, 2203–2208.
Bubici, G. 2018. Streptomyces spp. as biocontrol agents against Fusarium species. CAB Reviews 13, No. 050.
Burgess, LW., Knight, TE., Tesoriero, L. and Phan Thuy Hien. 2009. Diagnostic manual for plant diseases in Vietnam. Australian Center for International Agricultural Research – ACIAR (In Vietnamese)
Chemeltorit PP, Mutaqin KH, Widodo W. 2017. Combining Trichoderma hamatum THSW13 and Pseudomonas aeruginosa BJ10–86: a synergistic chili pepper seed treatment for Phytophthora capsici infested soil. Eur J Plant Pathol.147(1):157–166.
Cheng, X., X. Ji, Y. Ge, J. Li, W. Qi and K. Qiao. 2019. Characterization of Antagonistic Bacillusmethylotrophicus isolated from rhizosphere and its biocontrol effects on maize stalk rot. Phytopathology,109 (4): 571-581.
Chung S., Kong H., Buyer JS., Lakshman DK., Lydon J., Kim SD. 2008. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl Microbiol Biotechnol, 80 (1), 115-123
Nguyen Thi Kim Cuc, Tran thi Hong, Pham Thi Thuy Hoai, Pham Viet Cuong. 2014. Isolation of microorganisms antagonistic to plant pathogen fungi and evaluation the biological activity invitro. Science and technology journal 52 (4), 419-430 (In Vietnamese with English abstract)
Pham Viet Cuong, Pham van Toan, Nguyen thi Tuyet Mai, Nguyen Thi Kim Cuc. 2002. Indol-acetic-acid production of Bacillus isolation from soil in Vietnam. Proceeding of national conference on the biotechnology, Hanoi 12/2003, 213-217 (In Vietnamese with English abstract)
Damalas and Koutroubas, SD. 2018. Current Status and Recent Developments in Biopesticide Use. Agriculture 2018, 8, 13; doi:10.3390/agriculture8010013
Department of Crop Production. 2021. Summary report of 2021 and implementation of the 2022 plan (In Vietnamese).
Department of Plant Protection. 2010. List of harmful organisms on some crops and post-harvest plant products in Vietnam. Agricultural publishing house 2010 (In Vietnamese)
Department of Plant Protection. 2019. Report on the current production and proposal for sustainable pepper production in Vietnam (In Vietnamese)
Erwin, D. C., and Ribeiro, O. K. 1996. Phytophthora Diseases Worldwide. American Phytopathological Society Press, St. Paul, MN.
Ezziyyani, M., Requena, ME., Egea-Gilabert, C., Candela, ME. 2007. Biological Control of Phytophthora Root Rot of Pepper Using Trichoderma harzianum and Streptomyces rochei in Combination. J Phytopathol. junho de 155(6):342–349.
Gordon T.R. 2017. Fusarium oxysporum and the Fusarium wilt syndrome. Annual Review of Phytopathology 55(1):23–39.
Grosch R., Dealtry S., Schreiter S., Berg G., Mendonça-Hagler L., Smalla K. 2012. Biocontrol of Rhizoctonia solani: complex interaction of biocontrol strains, pathogen and indigenous microbial community in the rhizosphere of lettuce shown by molecular methods. Plant Soil 361 343–357. 10.1007/s11104-012-1239
Hanh Tran Thi Phuong, San-Lang Wang, Van Bon Nguyen, Dinh Minh Tran, Dinh Sy Nguyen and Anh Dzung Nguyen. 2019. Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam. Agronomy 9, 714; doi:10.3390/agronomy9110714
Harman GE. 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96:190-194.
Henderson, CF. and Tilton, EW. 1955. Test with acaricides against the brow wheat mite. J. Econ.Entomol.48: 157-161Nguyen Viet Hiep, Nguyen Thu Ha. 2014. Study on the nematode trapping ability of some ring fungus strains isolated from coffee and pepper growing soils in Vietnam. Vietnam Journal of Agricultural Science and Technology, No. 4(50), p. 90 – 96 (In Vietnamese with English abstract)
Nguyen Viet Hiep, Nguyen Thu Ha, Tran Thi Thanh Thuy and Pham Van Toan. 2019. Isolation and selection of Arthrobotrys nematophagous fungi to control the nematodes on coffee and black pepper plants in Vietnam. Archives of Phytopathology and Plant Protection Volume 52 Numbers 7–8: 825-843
Ibrahim MAR, Srour HAM (2013). Saponins Suppress Nematode Cholesterol Biosynthesis and Inhibit Root Knot Nematode Development in Tomato Seedlings. Nat Prod Chem Res 2: 123 doi:10.4172/ 2329-6836.1000123.
Jiang Z. Q., Guo Y. H., Li S. M., Qi H. Y., Guo J. H. 2006. Evaluation of biocontrol effciency of Bacillus preparations and application methods against Phytophthora blight of bell pepper. Biological Control 36. 216–223.
Khan, T., Shadab, S., Afroz, R., Aziz, M.A. and Farooqui, M. 2011. Study of suppressive effects of biological agent fungus, natural organic compounds and carbofuron on root knot nematodes of tomato (Lycopersicon esculentum). J Microbiol Biotechnol Rev 1, 7– 11.
Le Duc Khanh. 2015. Report on scientific and technological results of the project “Research on nematode damaged pepper, coffee plant and technology for the effective control in the main cultivation area“ (In Vietnamese).
Keswani, C., Singh, S.P. and Singh, H.B. 2013. A superstar in biocontrol enterprise: Trichoderma spp. Biotech Today 3, 27–30.
Keswani C., Mishra S., Sarma B.K., Singh S.P. and Singh HB. 2014. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Applied Microbiology and Biotechnology 98, 533–544.
Keswani C., Singh HB., García-Estrada C., Caradus J., He YW., Aichour SM., Glare TR., Borriss R., Sansinenea E. 2019. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Applied Microbiology and Biotechnology.
Kiewnick S. 2007. Practicalities of developing and registering microbial biological control agents. CAB Rev. 2 1–11. 10.1079/PAVSNNR20072013
Kumar S. and Singh A. 2015. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 2015, 6, e129
Dang Thuy Linh and Nguyen Van Hoa. 2004. Antagonistic activity and growth parameters of 3 trichoderma strains and 3 Phytophthora strains isolated from durian, longan trees. Scientific report of southern horticultural research institute (In Vietnamese with English abstract)
Tran Kim Loang. 2007. Study on production of Trichoderma preparation to controlling some pathogen fungi damaged Cacao, durian and pepper in highland Taynguyen. Scientific report of Western Highlands Agriculture and Forestry Science Institute (In Vietnamese with English abstract) .
Mardanova, A.M., Hadieva, G.F., Lutfullin, M.T., Khilyas, I.V., Minnullina, L.F., Gilyazeva, A.G., Bogomolnaya, L.M. and Sharipova, M.R. 2017. Bacillus subtilis strains with antifungal activity against the phytopathogenic Fungi. Agricultural Sciences, 8, 1-20.
Vu Thuy Nga. 2008. Isolation and selection of Bacillus antagonistic to Fusarium oxysporum damaged vegetable. Agricultural science and technology journal (In Vietnamese with English abstract)
Le Van Nhuong, Nguyen Van Cach, Quan Le Ha, Tran Lien Ha, Nguyen Thanh Hang, Hoang Dinh Hoa, Nguyen Lan Huong, Ngo Thi Mai, Dinh Kim Nhung, Khuat Huu Thanh, Nguyen Quang Thao, Pham Thi Thuy, Pham van Toan. 2009. Basic of biotechnology. Vol 4. Microbial technology. Education publishing house (In Vietnamese)
Niu,.XM. and Ke-Qin Zhang, KQ. 2011. Arthrobotrys oligospora: a model organism for understanding the interaction between fungi and nematodes, Mycology, 2:2, 59-78, DOI: 10.1080/21501203.2011.562559
Olson S. 2015. An analysis of the biopesticide market now and where is going. Outlooks Pest Manag. 26, 203–206.
Ramírez-Delgado, E., Luna-Ruíz1, J. J., Moreno-Rico, O., Quiroz-Velásquesz, J. D. C. and Hernández-Mendoza, J. L. 2018. Effect of Trichoderma on Growth and Sporangia Production of Phytophthora capsici. Journal of Agricultural Science, Vol. 10, No. 6, 8-15
Siahmoshteh, F., Z. Hamidi-Esfahani, D. Spadaro, M. Shams-Ghahfarokhi and M. Razzaghi-Abyaneh. 2019. Unraveling the mechanism of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens against aflatoxigenic Aspergillus parasiticus. 89: 300-307.
Siddiqui, Z. A. and Akhtar, M. S. 2009. Effects of antagonistic fungi and plant growth-promoting rhizobacteria on growth of tomato and reproduction of the root-knot nematode, Meloidogyne incognita. Australasian Plant Pathology, 2009, 38, 22–28.
Simon, S. and Anamika. 2011. Management of root knot diseases in rice caused by Meloidogyne graminicola through nematophagous fungi. Indian J Agric Sci 3, 122– 127.
Singh, U.B., Sahu, A.,Sahu,N., Singh, R.K. Renu, S., Singh, D.P., Manna, M.C., Sarma, B.K., Singh, H.B.,Singh, K.P. 2012. Arthrobotrys oligospora mediated biological control of diseases of tomato (Lycopersicon esculentum Mill.) caused by Meloidogyne incognita and Rhizoctonia solani. https://doi.org/10.1111/jam.12009.
Singh, U.B., Sahu, A., Sahu, N. 2013. Nematophagous fungi: Catenaria anguillulae and Dactylaria brochopaga from seed galls as potential biocontrol agents of Anguina tritici and Meloidogyne graminicola in wheat (Triticum aestivum L.). Biological Control, 67: 475-482.
Singh, H.B., Singh, A. and Sarma, B.K. 2014. Trichoderma viride 2 % WP (Strain No. BHU-2953) formulation suppresses tomato wilt caused by Fusarium oxysporum f. sp. lycopersici and chilli damping-off caused by Pythium aphanidermatum effectively under different agroclimatic conditions. International Journal of Agriculture, Environment and Biotechnology 7, 313–320.
Singh V., Ray S., Bisen K., Keswani C., Upadhyay RS., Sarma BK., Singh HB. 2017. Unravelling the Dual Applications of Trichoderma spp. as Biopesticide and Biofertilizer. In Advances in PGPR Research eds HB. Singh, BK. Sarma and C. Keswani. CAB International 2017, 264-274
Dinh Hong Thai and Le Minh Tuong. 2016. Survey the capability of streptomyces antagonism to Phytophthora sp. Damaged lottus plant. Scientific journal of Cantho university, 20-27 (In Vietnamese with English abstract)
Ha Minh Thanh. 2017. Report on scientific and technological results of the project “Improving production technology and application of probiotics to prevent fast and slow death on pepper plants (In Vietnamese)
Truong Thanh Thao, Vo Quoc Canh, Nguyen Thi Thu Nga. 2019. Isolation and selection of potential streptomyces strains antagonistic to Pratylenchus sp. In the laboratory. Scientific journal of Cantho university 55(2B), 19-27 (In Vietnamese with English abstract)
Nguyen Tang Ton, Nguyen Thi Huong, Le Quang Nhut, Nguyen Mong, Do Trung Binh, Nguyen Luong Thien, Do Dinh Dan, La Pham Lan, Doan Van Trung, Tran Kim Loang, Tran thi Thu Ha. 2010. Report on scientific and technological results of the project “Research on the intergrated management of soilborn diseases on the pepper” (In Vietnamese)
Trinh Thi Huyen Trang, San‑Lang Wang, Van Bon Nguyen, Minh Dinh Tran, Chien Thang Doan, Thi Phuong Khanh Vo, Que V. Huynh, Anh Dzung Nguyen. 2019. A potent antifungal rhizobacteria Bacillus velezensis RB.DS29 isolated from black pepper (Piper nigrumL.). Research on Chemical Intermediates 45:5309–5323, https://doi.org/ 10.1007/s11164-019-03971-5
Le Van Trinh. 2010. Report on scientific and technological results of the project “Research on the development of biopreparation to control pathogen fungi and nematodes damaged pepper” (In Vietnamese)
Tsay T. T., Chen P. C., Wu, W. S. 2006. A New Method for Isolating and Selecting Agents with High Antagonistic Ability Against Plant Parasitic Nematodes. Plant Pathology Bulletin 15: 9-16.
Usman, A. and Siddiqui, M.A. 2012. Effect of some fungal strains for the management of root-knot nematode (Meloidogyne in-cognita) on eggplant (Solanum melongena). Journal of Agriculture Tech-nology, 8(1): 213-218.
Wen-Chuan Chung, Rey-Shung Wu, Chia-Ping Hsu, Hung-Chang Huang, Jenn-Wen Huang. 2011. Application of antagonistic rhizobacteria for control of Fusarium seedling blight and basal rot of lily. Australasian Plant Pathol. (2011) 40:269–276
Zhang, K.Q., Hyde. K.D. 2014. Nematode-Trapping Fungi. Fungal Diversity Research Series. Volume 23. Springer Dordrecht Heidelberg New York London.