ABSTRACT
The Ministry of Agriculture, Forestry and Fisheries, Japan has set a strategy called "Green Food System Strategy" in 2021 and has announced the goal of 30% reduction of the amounts of chemical fertilizers use by 2050. To achieve a 30% reduction in chemical fertilizer use, crops need to efficiently absorb and utilize the applied chemical fertilizers in their roots. For this purpose, it is necessary to inoculate crops with biofertilizer microorganisms to promote rooting of the crops. However, there is no description on how to develop the use of biofertilizers. The Japanese government does not authorize most of biofertilizers except for VA mycorrhiza. However, the VA mycorrhiza is an ordinance-designated soil conditioner in Japan. Production of the VA mycorrhiza was 5 tons in Japan in 2020. The Tokachi Federation of Agricultural Cooperatives (TFAC: Japanese abbreviation: Tokachi Nokyoren) in Hokkaido is the major organization producing and distributing the rhizobium biofertilizers in Japan. In TFAC, 3 kinds of biofertilizers are currently produced and sold. “Mamezo” is a normal type of biofertilizer for soybeans, azuki beans and phaseolus beans. Rhizobium and Bradyrhizobium are mixed with peat and the natural organic matters. The second type of biofertilizer is “R-Processing Seeds.” This means leguminous seeds inoculated with rhizobia. The third is the “Hyper Coating Seeds.” In hyper coating seeds, leguminous grass seeds are coated with rhizobia within the capsule of calcium carbonate. In recent years, soybean cultivation has been recommended as a crop replacement for paddy fields. When growing soybeans in paddy fields, soils with high clay content cause drainage problems. Therefore, a method was developed in which deep-rooted hairy vetch is sown in the paddy field in the autumn after the rice harvest, and in the spring of the following year, the roots of the grown hairy vetch improve the drainage of the paddy field. Hairy vetch was an imported crop and did not grow naturally in Japan. Therefore, a rhizobium inoculant for hairy vetch was developed using root nodule bacteria distributed in Japan. Asian countries use biofertilizers for paddy rice using Azospirillum bacteria, but there was no biofertilizer for paddy rice in Japan. Dr. Yokoyama and his collaborators successfully developed a novel biofertilizer for paddy rice using spores of Bacillus pumilus TUAT1 strain.
Keywords: Biofertilizer, Bradyrhizobium, VA mycorrhiza, Rhizobium, Hairy vetch, Bacillus pumilus TUAT1
INTRODUCTION
The world population, which increased from 1.5 billion to 6.1 billion in the 20th century, has exceeded 8 billion this November, 2022. In 2019, Asia had a population of at least 4.6 billion. Sustaining these populations require increased food production. However, the stable supply of food and the sustainable development of agricultural production must be compatible with the issue of how to reduce the increase in carbon dioxide concentration that causes global climate change. Currently, most chemical nitrogen fertilizers are produced by the Haber-Bosch process, which converts nitrogen gas into ammonia. This manufacturing process uses a large amount of fossil energy and emits a large amount of carbon dioxide that induces global warming.
Reducing these emissions requires reducing the use of chemical nitrogen fertilizers while maintaining sustainable food production. The most promising way is developing agricultural microorganisms (having abilities as plant nutrition suppliers) into biofertilizers. Biofertilizer is a substance which contains living microorganisms which, when applied to seeds, plant surfaces, or soils, colonizes the rhizosphere or the interior of the plant and promotes growth by increasing the supply or availability of primary nutrients to the host plant (Vessey, J.K. 2003).
Looking at Japanese agricultural environments, the Japanese population continues to decline, and the number of farmers is also declining and aging. As a result, the domestic market is also shrinking. Therefore, to sustainably develop Japanese agriculture, it is necessary to cultivate overseas markets, such as expanding exports of agricultural products.
The Ministry of Agriculture, Forestry and Fisheries, Japan has set a strategy called "Green Food System Strategy" in 2021 and has announced the goal of 30% reduction of the amounts of chemical fertilizers use by 2050. To achieve this goal, it states that the optimization of the amount of fertilizer applied, the improvement of the utilization efficiency of the applied fertilizer, and the effective use of domestic resources such as livestock manure can replace chemical fertilizers. Specific strategies also include increasing knowledge of rhizosphere soil microbes to meet chemical fertilizer reduction targets.
To achieve a 30% reduction in chemical fertilizer use, crops need to efficiently absorb and utilize the applied chemical fertilizers in their roots. For this purpose, it is necessary to inoculate crops with biofertilizer microorganisms to promote rooting of the crops. However, there is no description on how to develop the use of biofertilizers.
This paper introduces the status of development and use of biofertilizers in Japan.
COMMERCIAL PRODUCTION AND USE OF BIOFERTILIZERS IN JAPAN
The Ministry of Agriculture, Forestry and Fisheries, Japan does not have a registration system for microbial materials such as biofertilizers. However, the government only controls the quality of mycorrhizal fungi and records the annual production volume. As for other microbial materials, there is no statistical record of their annual production volumes, so it is completely unknown how much they are produced in a year. Various companies sell microbial materials intended to increase the growth and yield of crops, but whether they are effective have not been evaluated by public agricultural research institutes.
This part describes only microbial materials that have been tested by public agricultural research institutes and have been confirmed to have inoculation effects such as growth promotion on crops.
(i) Vesicular-arbascular mycorrhizae inoculants
The beneficial effects of mycorrhizae on plant growth are well documented. Most importantly, it increases the uptake of immobile P from the soil. The predominant mycorrhizal associations that influence root P absorption involve the vesicular-arbascular mycorrhizae. VA mycorrhizae are the most abundant mycorrhizae and they colonize roots of a wide range of plant species in almost all types of soils. They form extensive hyphal networks in the soil and within root cortical tissues, forming intimate associations with the plasma membrane but not penetrating the cells (Leon V. Kochian, 2000).
The Japanese government does not authorize biofertilizers except for VA mycorrhiza. However, the VA mycorrhiza is an ordinance-designated soil conditioner in Japan (Table 1). Production of the VA mycorrhiza was 25 tons in Japan in 2011. In recent years, the production volume has been around 6 tons. Several companies supply VA mycorrhiza inoculants with carrier. The price of inoculants at 1kg is around US$100.
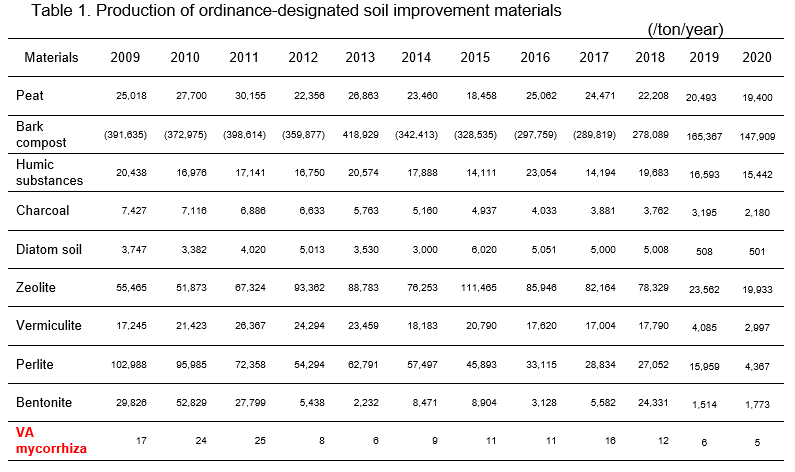
Figs. 1 and 2 show 2 kinds of commercial inoculants for strawberry and leek, respectively. In strawberry, the inoculants promoted growth and flowering.

(ii) Bradyrhizobium inoculants for soybean cultivations
Soybean [Glycine max L. (Merrill)] is a major leguminous crop worldwide, and it is extensively cultivated from eastern Asia to southeastern Asia. The genus Bradyrhizobium is a slow-growing, gram negative soil bacteria and is a major symbiont of soybeans. Based on 16S rRNA gene sequences, the genus Bradyrhizobium was classified into a clade in the Proteobacteria along with oligotrophic soil or aquatic bacteria such as Rhodopseudomonas palustris, Rhodoplanes roseus, Nitrobacter winogradskyi, Blastobacter denitrificans, and the pathogen Afipia spp. (Saito et al., 1998; Sawada et al., 2003; van Berkum and Eardly, 2002; Willems et al., 2001). The genus Bradyrhizobium currently consists of Bradyrhizobium japonicum (Jordan, 1982), B. elkanii (Kuykendall et al., 1992), B. liaoningense (Xu et al., 1995), B. yuanmingense (Yao et al., 2002), B. betae (Rivas et al., 2004), and B. canariense (Vinuesa et al., 2004). Under appropriate environmental conditions, soybean and Bradyrhizobium can initiate a symbiotic interaction, resulting in the development of nitrogen-fixing root nodules.
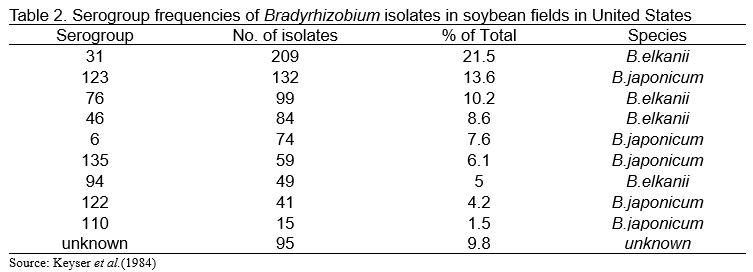
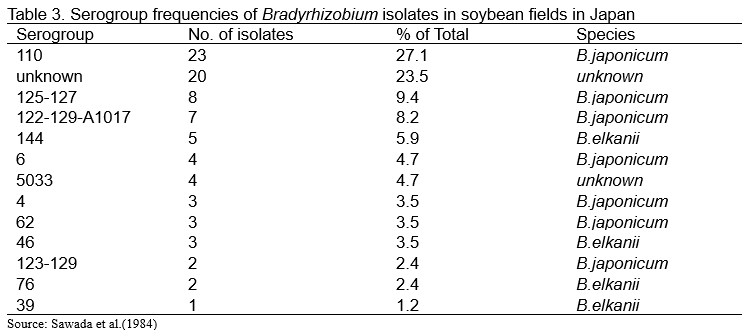
Genus Bradyrhizobium is known to display a large degree of antigenic heterogeneity, and can be categorized into several serological groups, based on differences in somatic antigens. Date and Decker (1965) classified the United State indigenous Bradyrhizobium strains into a total of 17 serological groups and 24 antigens. Thereafter, Keyser et al. (1984) reported that serological characterization of 972 isolates of Bradyrhizobium obtained from 65 soybean field locations in 12 states. The most predominant serogroup was 31 (21.5%), followed by 123 (13.6%) (Table 2). Sawada et al. (1989) used antisera prepared against USDA Bradyrhizobium strains to determine serological properties of Japanese isolates (Table 3). In case of Japanese soybean fields, the most predominant serogroup was 110 (27.1%), while frequency of serogroup 110 in US soybean fields was 1.5% (Table 2). We know that B. japonicum USDA 110 is applied to soybean fields worldwide as an effective inoculant. These results show that effective isolates categorized into serogroup 110 are widely distributed in Japanese soybean fields. Under this circumstance, farmers generally do not apply Bradyrhizobium inoculants to soybean at middle and southern part of Japan. However, at northern part of Japan (Hokkaido area), many farmers apply Bradyrhizobium inoculants to soybean.
The Tokachi Federation of Agricultural Cooperatives (TFAC: Japanese abbreviation: Tokachi Nokyoren) in Hokkaido, is the major organization producing and distributing the rhizobium biofertilizers in Japan. The TFAC is in Tokachi-Obihiro area in Hokkaido, the most northern prefecture in Japan, and it started the rhizobium biofertilizer business since 1953. In TFAC, 3 kinds of biofertilizers are produced and sold presently.
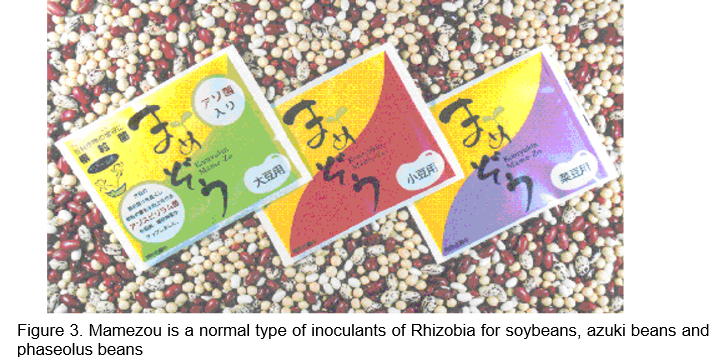
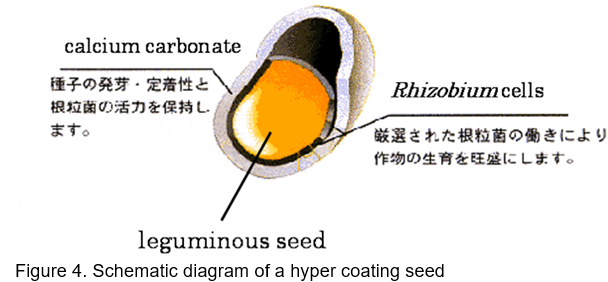
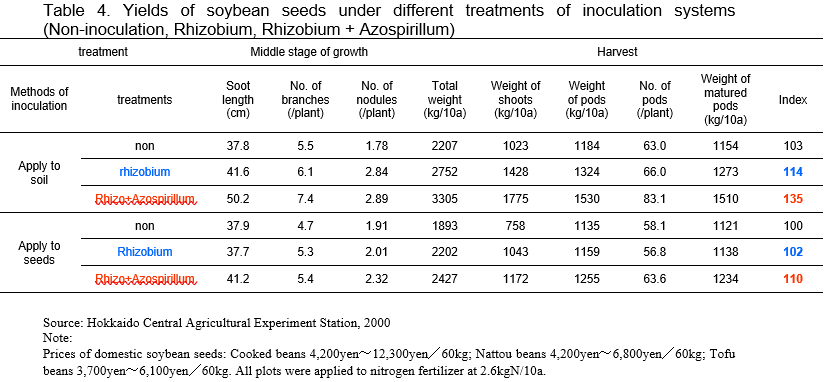
“Mamezo” is a normal type of biofertilizer for soybeans, azuki beans and phaseolus beans (Fig. 3). Rhizobium and Bradyrhizobium are mixed with peat and natural organic matters. The second type of biofertilizer is “R-Processing Seeds.” This means leguminous seeds inoculated with rhizobia. The final type of biofertilizer is “Hyper Coating Seeds” (Fig.4). In hyper coating seeds, leguminous grass seed are coated with rhizobia within the capsule of calcium carbonate. These biofertilizers are being used by about 80 % of farmers in Hokkaido. TFAC shows that this biofertilizer enhances legume growth, produces high seed yield and high nutritional quality (protein-rich). In the field of soybean cultivation with crop rotation system, 1.2 times of nodulation is possible and about 4 % increase of soybean yield is substantiated by inoculation compared with non-inoculated case on the average. The TFAC facility with two production lines for producing the concentrated Rhizobium inoculant paste was completed in 1990. Microorganisms are cultured and propagated with sucrose and concentrated by a centrifuge, and then frozen for storage. The frozen culture is thawed and mixed with sterilized peat (carrier) to produce biofertilizers. TFAC uses peat imported from Canada as biofertilizer carrier and acetylene reduction method is adopted to measure the N2 fixation efficiency. The selling price of “Mamezo” is about US$5/40g pack for 10a while the cost of microorganism itself is about US$0.6. Furthermore, mix–inoculation treatment with Bradyrhizobium and Azospirillum to soybean showed 35% increase in soybean seeds production compared with that of treatment without inoculants in field test conducted in 2000 (Table 4, http://www.agri.pref.hokkaido.jp/center/kenkyuseika/gaiyosho/h13gaiyo/2001304.htm). If the increase is replicable in farmers’ fields, the inoculants containing rhizobium and Azospirillum are promising biofertilizers for soybean cultivation in Hokkaido region.
(iii) Hairy vetch root nodule bacteria for soybean crop cultivation in padAll
In recent years, soybean cultivation has been recommended as a crop replacement for paddy fields. When growing soybeans in paddy fields, soils with high clay content cause drainage problems. Especially in paddy fields with poor drainage, soybean growth is extremely suppressed. Therefore, a method was developed in which deep-rooted hairy vetch is sown in the paddy field in the autumn after the rice harvest, and in the spring of the following year, the roots of the grown hairy vetch improve the drainage of the paddy field (Fig. 5). In addition, hairy vetch is incorporated into the soil as a green manure prior to soybean cultivation. With its green manure effect, a technique was developed to increase the yield of soybeans without using chemical nitrogen fertilizers.
Hairy vetch was an imported crop and did not grow naturally in Japan. Therefore, when the hairy vetch was introduced into the paddy field, the growth of the hairy vetch was extremely poor. Dr. Takashi Sato of Akita Prefectural University searched for and evaluated root nodule bacteria in the soil where hairy vetch was cultivated, discovered root nodule bacteria suitable for cultivation in Japan, and started selling it as a root nodule biofertilizer for hairy vetch (Fig. 6, 7, and 8).




FUTURE DIRECTION OF BIOFERTILIZERS IN JAPAN
(i) Problems in dissemination of biofertilizers in Asia
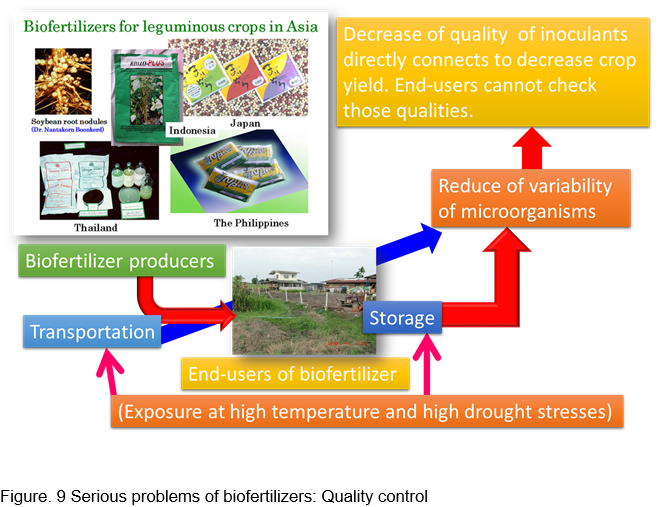
Biofertilizers are considered to contribute to sustainable agriculture. In those biofertilizers, living microorganisms promote supply of nutrients to crops, therefore, those microorganisms need to keep their activities just before applying the fields.
Biofertilizers produced by biofertilizer producers are transported to farmers as end users. In this process, those biofertilizers are exposed to high temperature. Furthermore, in farmer's storage, those biofertilizers are also exposed to high ambient temperature. Cell number of beneficial bacteria in biofertilizers which have been exposed to high temperature rapidly decrease and this leads to degradation of their quality.
When using biofertilizers that have failed quality control, the growth promotion effect of the biofertilizer is hardly obtained. As a result, farmers who purchase those biofertilizers cannot see the effect of promoting growth, and the yield does not increase. Farmers with such experiences never use biofertilizers again. The agricultural market also sells many microbial materials with little visible effect. As a result, many farmers have lost confidence in microbial materials.
(ii) Risk management of Biofertilizers in Japan
In Japan, some companies that sell biofertilizers have strict quality control. For example, Nefueru is produced by Tokachi Federation of Agricultural Cooperatives (TFAC: Japanese abbreviation: Tokachi Nokyoren) in Hokkaido, which is the major organization producing and distributing the rhizobium biofertilizers in Japan.

TFAC conducts strict quality control with the following procedures:
- Quality holding period of Nefueru is 3 months in cool condition at 10 oC;
- After every 3 months, TFAC recalls the materials to avoid clams of farmers; and
- Thus, quality control is very important to disseminate biofertilizers to farmers.
Therefore, when selecting the microorganisms for biofertilizers, we need to pay attention to not only the high nutrient supply abilities to a target but also stress resistances such as high temperature and drought considering the dissemination process of biofertilizers.
(iii) A novel biofertilizer for paddy rice: R/D of Bacillus biofertizer in TUAT
So far, there is no biofertilizer for rice cultivation to reduce use of chemical nitrogen fertilizer in Japan. Around 15 years ago, we started to develop biofertilizers for rice cultivation in Japan.
In many Asian countries, they are developing biofertilizers for rice using Azospirillum. However, Azospirillum is very difficult to control quality such as survival ratio of bacteria in bags. While there is no report of Bacillus biofertilzer for rice. Furthermore, Bacillus is spore forming bacteria, spore cells have high environmental stress resistance. Under these conditions, we developed Bacillus biofertilizer “Kikuichi” for paddy rice (Fig. 11).
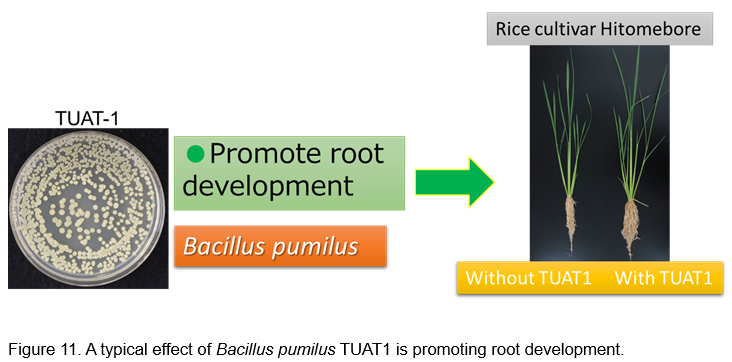
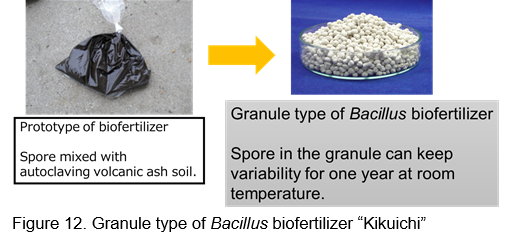
This biofertilizer is a granule type, and spores of Bacillus pumilus TUAT1 are absorbed by the granule having base material of zeolite. Size of granule is around 3 mm and 107 spores keep in one gram of granules. Quality of this biofertilizer can keep at least 1 year at room temperature (Fig. 12).
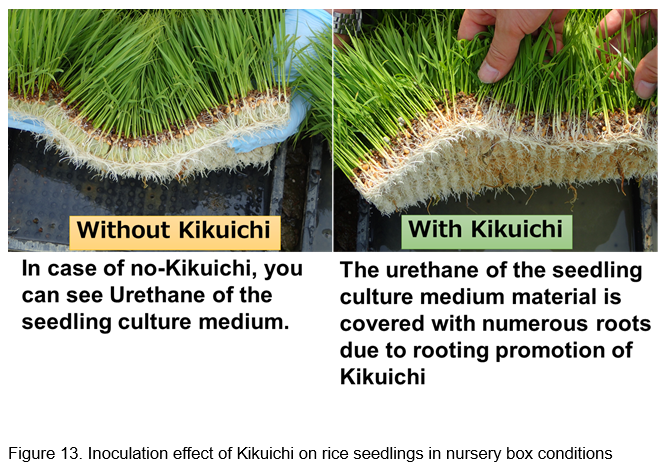
When Bacillus biofertilizer “Kikuichi” applies to rice seeds in nursery boxes, the seedlings show over 30% of increase of root growth such as root number and root weight of rice seedling against the control plants without “Kikuichi” (Fig. 13).
When the rice plants with “Kikuichi” are transplanted to paddy fields, root growths occur in paddy fields which promote nitrogen fertilizer uptake. These inoculation effects induce an increase of tillers of treated rice plants, therefore, the application of “Kikuichi” increase yields of brown rice at 10 to 30%. Rice plants which are applied with “Kikuichi” promote an efficiency of fertilizer utilization to develop root systems, therefore this property directly connects to reduce amounts of fertilizer application.
REFERENCES
Date, R. A. and A. M. Decker. 1963. Minimal antigenic constitution of 28 strains of Rhizobium japonicum. Can. J. Microbiol. 11:1-8.
Jordan, D. C. 1982. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, and root nodule bacteria from leguminous plants. Int. J. Syst. Bacteriol. 32: 136-139.
Keyser H. H., D. F. Weber, and S. L. Uratsu. 1984. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 47:613-615.
Kuykendall, L. D., Saxena, B., Devine, T. E., and Udell, S. E. 1992. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can. J. Microbiol. 38: 501-505.
Leon V. Kochian. 2000. Molecular physiology of mineral nutrient acquisition, transport. and utilization. In Biochemistry & Molecular biology of plant ed. Buchanan et al. P.1204-1249. America society of plant physiologists
Rivas, R., Willems, A., Palomo, J.L., Garcia-Benavides, P., Mateos, P.F., Martinez-Molina, E., Gillis, M., and Velazquez, E. 2004. Bradyrhizobium betae sp. nov., isolated from roots of Beta vulgaris affected by tumour-like deformations. Int J Syst Evol Microbiol. 54: 1271-1275.
Saito, A., Mitsui, H., Hattori, R., Minamisawa, K., and Hattori, T. 1998. Slow-growing and oligotrophic soil bacteria phylogenetically close to Bradyrhizobium japonicum. FEMS Microbial. Ecol. 25: 277-286.
Sawada, H., Kuykendall, L.D, and Young, J.M. 2003. Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. J Gen Appl Microbiol. 49: 155-179.
Sawada, Y., Miyashita, K., Tanabe, I., and Kato, K. 1989. Hup phenotype and serogroup identity of soybean-nodulating bacteria isolated from Japanese soil. Soil Sci. Plant Nutr. 35:281-288.
Van Berkum, P., and Eardly, B.D. 2002. The aquatic budding bacterium Blastobacter denitrificans is a nitrogen-fixing symbiont of Aeschynomene indica. Appl Environ Microbiol. 68: 1132-1136.
Vessey, J.K. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil 255: 571-586
Vinuesa, P., Leon-Barrios, M., Silva, C., Willems, A., Jarabo-Lorenzo, A., Perez-Galdona, R., Werner, D., and Martinez-Romero, E. 2005. Bradyrhizobium canariense sp. nov., an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int J Syst Evol Microbiol. 55: 569-575.
Willems, A., Coopman, R., and Gillis, M. 2001. Phylogenetic and DNA-DNA hybridization analyses of Bradyrhizobium species. Int J Syst Evol Microbiol. 51: 111-117.
Xu, L. M., Ge, C., Cui, Z., Li, J., and Fan, H. 1995. Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int. J. Syst. Bacteriol. 45: 706-711.
Yao, Z.Y., Kan, F.L., Wang, E.T., Wei, G.H., and Chen, W.X. 2002. Characterization of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int J Syst Evol Microbiol. 52: 2219-2230
Development and Use of Biofertilizers in Japan: Current Situation and Future Directions
DOI: https://doi.org/10.56669/TGJS1978
ABSTRACT
The Ministry of Agriculture, Forestry and Fisheries, Japan has set a strategy called "Green Food System Strategy" in 2021 and has announced the goal of 30% reduction of the amounts of chemical fertilizers use by 2050. To achieve a 30% reduction in chemical fertilizer use, crops need to efficiently absorb and utilize the applied chemical fertilizers in their roots. For this purpose, it is necessary to inoculate crops with biofertilizer microorganisms to promote rooting of the crops. However, there is no description on how to develop the use of biofertilizers. The Japanese government does not authorize most of biofertilizers except for VA mycorrhiza. However, the VA mycorrhiza is an ordinance-designated soil conditioner in Japan. Production of the VA mycorrhiza was 5 tons in Japan in 2020. The Tokachi Federation of Agricultural Cooperatives (TFAC: Japanese abbreviation: Tokachi Nokyoren) in Hokkaido is the major organization producing and distributing the rhizobium biofertilizers in Japan. In TFAC, 3 kinds of biofertilizers are currently produced and sold. “Mamezo” is a normal type of biofertilizer for soybeans, azuki beans and phaseolus beans. Rhizobium and Bradyrhizobium are mixed with peat and the natural organic matters. The second type of biofertilizer is “R-Processing Seeds.” This means leguminous seeds inoculated with rhizobia. The third is the “Hyper Coating Seeds.” In hyper coating seeds, leguminous grass seeds are coated with rhizobia within the capsule of calcium carbonate. In recent years, soybean cultivation has been recommended as a crop replacement for paddy fields. When growing soybeans in paddy fields, soils with high clay content cause drainage problems. Therefore, a method was developed in which deep-rooted hairy vetch is sown in the paddy field in the autumn after the rice harvest, and in the spring of the following year, the roots of the grown hairy vetch improve the drainage of the paddy field. Hairy vetch was an imported crop and did not grow naturally in Japan. Therefore, a rhizobium inoculant for hairy vetch was developed using root nodule bacteria distributed in Japan. Asian countries use biofertilizers for paddy rice using Azospirillum bacteria, but there was no biofertilizer for paddy rice in Japan. Dr. Yokoyama and his collaborators successfully developed a novel biofertilizer for paddy rice using spores of Bacillus pumilus TUAT1 strain.
Keywords: Biofertilizer, Bradyrhizobium, VA mycorrhiza, Rhizobium, Hairy vetch, Bacillus pumilus TUAT1
INTRODUCTION
The world population, which increased from 1.5 billion to 6.1 billion in the 20th century, has exceeded 8 billion this November, 2022. In 2019, Asia had a population of at least 4.6 billion. Sustaining these populations require increased food production. However, the stable supply of food and the sustainable development of agricultural production must be compatible with the issue of how to reduce the increase in carbon dioxide concentration that causes global climate change. Currently, most chemical nitrogen fertilizers are produced by the Haber-Bosch process, which converts nitrogen gas into ammonia. This manufacturing process uses a large amount of fossil energy and emits a large amount of carbon dioxide that induces global warming.
Reducing these emissions requires reducing the use of chemical nitrogen fertilizers while maintaining sustainable food production. The most promising way is developing agricultural microorganisms (having abilities as plant nutrition suppliers) into biofertilizers. Biofertilizer is a substance which contains living microorganisms which, when applied to seeds, plant surfaces, or soils, colonizes the rhizosphere or the interior of the plant and promotes growth by increasing the supply or availability of primary nutrients to the host plant (Vessey, J.K. 2003).
Looking at Japanese agricultural environments, the Japanese population continues to decline, and the number of farmers is also declining and aging. As a result, the domestic market is also shrinking. Therefore, to sustainably develop Japanese agriculture, it is necessary to cultivate overseas markets, such as expanding exports of agricultural products.
The Ministry of Agriculture, Forestry and Fisheries, Japan has set a strategy called "Green Food System Strategy" in 2021 and has announced the goal of 30% reduction of the amounts of chemical fertilizers use by 2050. To achieve this goal, it states that the optimization of the amount of fertilizer applied, the improvement of the utilization efficiency of the applied fertilizer, and the effective use of domestic resources such as livestock manure can replace chemical fertilizers. Specific strategies also include increasing knowledge of rhizosphere soil microbes to meet chemical fertilizer reduction targets.
To achieve a 30% reduction in chemical fertilizer use, crops need to efficiently absorb and utilize the applied chemical fertilizers in their roots. For this purpose, it is necessary to inoculate crops with biofertilizer microorganisms to promote rooting of the crops. However, there is no description on how to develop the use of biofertilizers.
This paper introduces the status of development and use of biofertilizers in Japan.
COMMERCIAL PRODUCTION AND USE OF BIOFERTILIZERS IN JAPAN
The Ministry of Agriculture, Forestry and Fisheries, Japan does not have a registration system for microbial materials such as biofertilizers. However, the government only controls the quality of mycorrhizal fungi and records the annual production volume. As for other microbial materials, there is no statistical record of their annual production volumes, so it is completely unknown how much they are produced in a year. Various companies sell microbial materials intended to increase the growth and yield of crops, but whether they are effective have not been evaluated by public agricultural research institutes.
This part describes only microbial materials that have been tested by public agricultural research institutes and have been confirmed to have inoculation effects such as growth promotion on crops.
(i) Vesicular-arbascular mycorrhizae inoculants
The beneficial effects of mycorrhizae on plant growth are well documented. Most importantly, it increases the uptake of immobile P from the soil. The predominant mycorrhizal associations that influence root P absorption involve the vesicular-arbascular mycorrhizae. VA mycorrhizae are the most abundant mycorrhizae and they colonize roots of a wide range of plant species in almost all types of soils. They form extensive hyphal networks in the soil and within root cortical tissues, forming intimate associations with the plasma membrane but not penetrating the cells (Leon V. Kochian, 2000).
The Japanese government does not authorize biofertilizers except for VA mycorrhiza. However, the VA mycorrhiza is an ordinance-designated soil conditioner in Japan (Table 1). Production of the VA mycorrhiza was 25 tons in Japan in 2011. In recent years, the production volume has been around 6 tons. Several companies supply VA mycorrhiza inoculants with carrier. The price of inoculants at 1kg is around US$100.
Figs. 1 and 2 show 2 kinds of commercial inoculants for strawberry and leek, respectively. In strawberry, the inoculants promoted growth and flowering.
(ii) Bradyrhizobium inoculants for soybean cultivations
Soybean [Glycine max L. (Merrill)] is a major leguminous crop worldwide, and it is extensively cultivated from eastern Asia to southeastern Asia. The genus Bradyrhizobium is a slow-growing, gram negative soil bacteria and is a major symbiont of soybeans. Based on 16S rRNA gene sequences, the genus Bradyrhizobium was classified into a clade in the Proteobacteria along with oligotrophic soil or aquatic bacteria such as Rhodopseudomonas palustris, Rhodoplanes roseus, Nitrobacter winogradskyi, Blastobacter denitrificans, and the pathogen Afipia spp. (Saito et al., 1998; Sawada et al., 2003; van Berkum and Eardly, 2002; Willems et al., 2001). The genus Bradyrhizobium currently consists of Bradyrhizobium japonicum (Jordan, 1982), B. elkanii (Kuykendall et al., 1992), B. liaoningense (Xu et al., 1995), B. yuanmingense (Yao et al., 2002), B. betae (Rivas et al., 2004), and B. canariense (Vinuesa et al., 2004). Under appropriate environmental conditions, soybean and Bradyrhizobium can initiate a symbiotic interaction, resulting in the development of nitrogen-fixing root nodules.
Genus Bradyrhizobium is known to display a large degree of antigenic heterogeneity, and can be categorized into several serological groups, based on differences in somatic antigens. Date and Decker (1965) classified the United State indigenous Bradyrhizobium strains into a total of 17 serological groups and 24 antigens. Thereafter, Keyser et al. (1984) reported that serological characterization of 972 isolates of Bradyrhizobium obtained from 65 soybean field locations in 12 states. The most predominant serogroup was 31 (21.5%), followed by 123 (13.6%) (Table 2). Sawada et al. (1989) used antisera prepared against USDA Bradyrhizobium strains to determine serological properties of Japanese isolates (Table 3). In case of Japanese soybean fields, the most predominant serogroup was 110 (27.1%), while frequency of serogroup 110 in US soybean fields was 1.5% (Table 2). We know that B. japonicum USDA 110 is applied to soybean fields worldwide as an effective inoculant. These results show that effective isolates categorized into serogroup 110 are widely distributed in Japanese soybean fields. Under this circumstance, farmers generally do not apply Bradyrhizobium inoculants to soybean at middle and southern part of Japan. However, at northern part of Japan (Hokkaido area), many farmers apply Bradyrhizobium inoculants to soybean.
The Tokachi Federation of Agricultural Cooperatives (TFAC: Japanese abbreviation: Tokachi Nokyoren) in Hokkaido, is the major organization producing and distributing the rhizobium biofertilizers in Japan. The TFAC is in Tokachi-Obihiro area in Hokkaido, the most northern prefecture in Japan, and it started the rhizobium biofertilizer business since 1953. In TFAC, 3 kinds of biofertilizers are produced and sold presently.
“Mamezo” is a normal type of biofertilizer for soybeans, azuki beans and phaseolus beans (Fig. 3). Rhizobium and Bradyrhizobium are mixed with peat and natural organic matters. The second type of biofertilizer is “R-Processing Seeds.” This means leguminous seeds inoculated with rhizobia. The final type of biofertilizer is “Hyper Coating Seeds” (Fig.4). In hyper coating seeds, leguminous grass seed are coated with rhizobia within the capsule of calcium carbonate. These biofertilizers are being used by about 80 % of farmers in Hokkaido. TFAC shows that this biofertilizer enhances legume growth, produces high seed yield and high nutritional quality (protein-rich). In the field of soybean cultivation with crop rotation system, 1.2 times of nodulation is possible and about 4 % increase of soybean yield is substantiated by inoculation compared with non-inoculated case on the average. The TFAC facility with two production lines for producing the concentrated Rhizobium inoculant paste was completed in 1990. Microorganisms are cultured and propagated with sucrose and concentrated by a centrifuge, and then frozen for storage. The frozen culture is thawed and mixed with sterilized peat (carrier) to produce biofertilizers. TFAC uses peat imported from Canada as biofertilizer carrier and acetylene reduction method is adopted to measure the N2 fixation efficiency. The selling price of “Mamezo” is about US$5/40g pack for 10a while the cost of microorganism itself is about US$0.6. Furthermore, mix–inoculation treatment with Bradyrhizobium and Azospirillum to soybean showed 35% increase in soybean seeds production compared with that of treatment without inoculants in field test conducted in 2000 (Table 4, http://www.agri.pref.hokkaido.jp/center/kenkyuseika/gaiyosho/h13gaiyo/2001304.htm). If the increase is replicable in farmers’ fields, the inoculants containing rhizobium and Azospirillum are promising biofertilizers for soybean cultivation in Hokkaido region.
(iii) Hairy vetch root nodule bacteria for soybean crop cultivation in padAll
In recent years, soybean cultivation has been recommended as a crop replacement for paddy fields. When growing soybeans in paddy fields, soils with high clay content cause drainage problems. Especially in paddy fields with poor drainage, soybean growth is extremely suppressed. Therefore, a method was developed in which deep-rooted hairy vetch is sown in the paddy field in the autumn after the rice harvest, and in the spring of the following year, the roots of the grown hairy vetch improve the drainage of the paddy field (Fig. 5). In addition, hairy vetch is incorporated into the soil as a green manure prior to soybean cultivation. With its green manure effect, a technique was developed to increase the yield of soybeans without using chemical nitrogen fertilizers.
Hairy vetch was an imported crop and did not grow naturally in Japan. Therefore, when the hairy vetch was introduced into the paddy field, the growth of the hairy vetch was extremely poor. Dr. Takashi Sato of Akita Prefectural University searched for and evaluated root nodule bacteria in the soil where hairy vetch was cultivated, discovered root nodule bacteria suitable for cultivation in Japan, and started selling it as a root nodule biofertilizer for hairy vetch (Fig. 6, 7, and 8).
FUTURE DIRECTION OF BIOFERTILIZERS IN JAPAN
(i) Problems in dissemination of biofertilizers in Asia
Biofertilizers are considered to contribute to sustainable agriculture. In those biofertilizers, living microorganisms promote supply of nutrients to crops, therefore, those microorganisms need to keep their activities just before applying the fields.
Biofertilizers produced by biofertilizer producers are transported to farmers as end users. In this process, those biofertilizers are exposed to high temperature. Furthermore, in farmer's storage, those biofertilizers are also exposed to high ambient temperature. Cell number of beneficial bacteria in biofertilizers which have been exposed to high temperature rapidly decrease and this leads to degradation of their quality.
When using biofertilizers that have failed quality control, the growth promotion effect of the biofertilizer is hardly obtained. As a result, farmers who purchase those biofertilizers cannot see the effect of promoting growth, and the yield does not increase. Farmers with such experiences never use biofertilizers again. The agricultural market also sells many microbial materials with little visible effect. As a result, many farmers have lost confidence in microbial materials.
(ii) Risk management of Biofertilizers in Japan
In Japan, some companies that sell biofertilizers have strict quality control. For example, Nefueru is produced by Tokachi Federation of Agricultural Cooperatives (TFAC: Japanese abbreviation: Tokachi Nokyoren) in Hokkaido, which is the major organization producing and distributing the rhizobium biofertilizers in Japan.
TFAC conducts strict quality control with the following procedures:
Therefore, when selecting the microorganisms for biofertilizers, we need to pay attention to not only the high nutrient supply abilities to a target but also stress resistances such as high temperature and drought considering the dissemination process of biofertilizers.
(iii) A novel biofertilizer for paddy rice: R/D of Bacillus biofertizer in TUAT
So far, there is no biofertilizer for rice cultivation to reduce use of chemical nitrogen fertilizer in Japan. Around 15 years ago, we started to develop biofertilizers for rice cultivation in Japan.
In many Asian countries, they are developing biofertilizers for rice using Azospirillum. However, Azospirillum is very difficult to control quality such as survival ratio of bacteria in bags. While there is no report of Bacillus biofertilzer for rice. Furthermore, Bacillus is spore forming bacteria, spore cells have high environmental stress resistance. Under these conditions, we developed Bacillus biofertilizer “Kikuichi” for paddy rice (Fig. 11).
This biofertilizer is a granule type, and spores of Bacillus pumilus TUAT1 are absorbed by the granule having base material of zeolite. Size of granule is around 3 mm and 107 spores keep in one gram of granules. Quality of this biofertilizer can keep at least 1 year at room temperature (Fig. 12).
When Bacillus biofertilizer “Kikuichi” applies to rice seeds in nursery boxes, the seedlings show over 30% of increase of root growth such as root number and root weight of rice seedling against the control plants without “Kikuichi” (Fig. 13).
When the rice plants with “Kikuichi” are transplanted to paddy fields, root growths occur in paddy fields which promote nitrogen fertilizer uptake. These inoculation effects induce an increase of tillers of treated rice plants, therefore, the application of “Kikuichi” increase yields of brown rice at 10 to 30%. Rice plants which are applied with “Kikuichi” promote an efficiency of fertilizer utilization to develop root systems, therefore this property directly connects to reduce amounts of fertilizer application.
REFERENCES
Date, R. A. and A. M. Decker. 1963. Minimal antigenic constitution of 28 strains of Rhizobium japonicum. Can. J. Microbiol. 11:1-8.
Jordan, D. C. 1982. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, and root nodule bacteria from leguminous plants. Int. J. Syst. Bacteriol. 32: 136-139.
Keyser H. H., D. F. Weber, and S. L. Uratsu. 1984. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 47:613-615.
Kuykendall, L. D., Saxena, B., Devine, T. E., and Udell, S. E. 1992. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can. J. Microbiol. 38: 501-505.
Leon V. Kochian. 2000. Molecular physiology of mineral nutrient acquisition, transport. and utilization. In Biochemistry & Molecular biology of plant ed. Buchanan et al. P.1204-1249. America society of plant physiologists
Rivas, R., Willems, A., Palomo, J.L., Garcia-Benavides, P., Mateos, P.F., Martinez-Molina, E., Gillis, M., and Velazquez, E. 2004. Bradyrhizobium betae sp. nov., isolated from roots of Beta vulgaris affected by tumour-like deformations. Int J Syst Evol Microbiol. 54: 1271-1275.
Saito, A., Mitsui, H., Hattori, R., Minamisawa, K., and Hattori, T. 1998. Slow-growing and oligotrophic soil bacteria phylogenetically close to Bradyrhizobium japonicum. FEMS Microbial. Ecol. 25: 277-286.
Sawada, H., Kuykendall, L.D, and Young, J.M. 2003. Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. J Gen Appl Microbiol. 49: 155-179.
Sawada, Y., Miyashita, K., Tanabe, I., and Kato, K. 1989. Hup phenotype and serogroup identity of soybean-nodulating bacteria isolated from Japanese soil. Soil Sci. Plant Nutr. 35:281-288.
Van Berkum, P., and Eardly, B.D. 2002. The aquatic budding bacterium Blastobacter denitrificans is a nitrogen-fixing symbiont of Aeschynomene indica. Appl Environ Microbiol. 68: 1132-1136.
Vessey, J.K. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil 255: 571-586
Vinuesa, P., Leon-Barrios, M., Silva, C., Willems, A., Jarabo-Lorenzo, A., Perez-Galdona, R., Werner, D., and Martinez-Romero, E. 2005. Bradyrhizobium canariense sp. nov., an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int J Syst Evol Microbiol. 55: 569-575.
Willems, A., Coopman, R., and Gillis, M. 2001. Phylogenetic and DNA-DNA hybridization analyses of Bradyrhizobium species. Int J Syst Evol Microbiol. 51: 111-117.
Xu, L. M., Ge, C., Cui, Z., Li, J., and Fan, H. 1995. Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int. J. Syst. Bacteriol. 45: 706-711.
Yao, Z.Y., Kan, F.L., Wang, E.T., Wei, G.H., and Chen, W.X. 2002. Characterization of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int J Syst Evol Microbiol. 52: 2219-2230